PinX1 localizes to telomeres and stabilizes TRF1 at mitosis
- PMID: 22331467
- PMCID: PMC3318592
- DOI: 10.1128/MCB.05641-11
PinX1 localizes to telomeres and stabilizes TRF1 at mitosis
Abstract
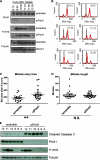
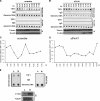
Human telomeres are DNA-protein complexes that cap and protect the ends of chromosomes. The protein PinX1 associates with telomeres through an interaction with the resident double-stranded telomere-binding protein TRF1. PinX1 also binds to and inhibits telomerase, the enzyme responsible for complete replication of telomeric DNA. We now report that endogenous PinX1 associates with telomeres primarily at mitosis. Moreover, knockdown of PinX1 caused delayed mitotic entry and reduced the accumulation of TRF1 on telomeres during this stage of the cell cycle. Taking these findings together, we suggest that one function of PinX1 is to stabilize TRF1 during mitosis, perhaps to promote transition into M phase of the cell cycle.
Figures



Similar articles
-
Telomerase inhibitor PinX1 provides a link between TRF1 and telomerase to prevent telomere elongation.J Biol Chem. 2011 Feb 4;286(5):3894-906. doi: 10.1074/jbc.M110.180174. Epub 2010 Nov 30. J Biol Chem. 2011. PMID: 21119197 Free PMC article.
-
PinX1, a telomere repeat-binding factor 1 (TRF1)-interacting protein, maintains telomere integrity by modulating TRF1 homeostasis, the process in which human telomerase reverse Transcriptase (hTERT) plays dual roles.J Biol Chem. 2014 Mar 7;289(10):6886-6898. doi: 10.1074/jbc.M113.506006. Epub 2014 Jan 10. J Biol Chem. 2014. PMID: 24415760 Free PMC article.
-
Human PinX1 mediates TRF1 accumulation in nucleolus and enhances TRF1 binding to telomeres.J Mol Biol. 2009 May 22;388(5):928-40. doi: 10.1016/j.jmb.2009.02.051. Epub 2009 Mar 3. J Mol Biol. 2009. PMID: 19265708
-
Role of Pin2/TRF1 in telomere maintenance and cell cycle control.J Cell Biochem. 2003 May 1;89(1):19-37. doi: 10.1002/jcb.10496. J Cell Biochem. 2003. PMID: 12682905 Review.
-
Post-translational modifications of TRF1 and TRF2 and their roles in telomere maintenance.Mech Ageing Dev. 2012 Jun;133(6):421-34. doi: 10.1016/j.mad.2012.05.002. Epub 2012 May 23. Mech Ageing Dev. 2012. PMID: 22634377 Review.
Cited by
-
Prognostic and Clinicopathological Value of PINX1 in Various Human Tumors: A Meta-Analysis.Biomed Res Int. 2018 Jul 16;2018:4621015. doi: 10.1155/2018/4621015. eCollection 2018. Biomed Res Int. 2018. PMID: 30079348 Free PMC article.
-
The truncated isoform of the receptor for hyaluronan-mediated motility (RHAMMΔ163) modulates shelterin and telomerase reverse transcriptase transcription affecting telomerase activity.Front Aging. 2025 Jun 30;6:1604051. doi: 10.3389/fragi.2025.1604051. eCollection 2025. Front Aging. 2025. PMID: 40661163 Free PMC article.
-
The 68-kDa telomeric repeat binding factor 1 (TRF1)-associated protein (TAP68) interacts with and recruits TRF1 to the spindle pole during mitosis.J Biol Chem. 2014 May 16;289(20):14145-56. doi: 10.1074/jbc.M113.526244. Epub 2014 Apr 1. J Biol Chem. 2014. PMID: 24692559 Free PMC article.
-
Integrating data and knowledge to identify functional modules of genes: a multilayer approach.BMC Bioinformatics. 2019 May 2;20(1):225. doi: 10.1186/s12859-019-2800-y. BMC Bioinformatics. 2019. PMID: 31046665 Free PMC article.
-
Cell cycle regulated phosphorylation of the telomere-associated protein TIN2.PLoS One. 2013 Aug 16;8(8):e71697. doi: 10.1371/journal.pone.0071697. eCollection 2013. PLoS One. 2013. PMID: 23977114 Free PMC article.
References
-
- Banik SS, Counter CM. 2004. Characterization of interactions between PinX1 and human telomerase subunits hTERT and hTR. J. Biol. Chem. 279: 51745–51748 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
