In vitro and in vivo antiangiogenic properties of the serpin protease nexin-1
- PMID: 22331468
- PMCID: PMC3318585
- DOI: 10.1128/MCB.06554-11
In vitro and in vivo antiangiogenic properties of the serpin protease nexin-1
Abstract
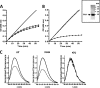
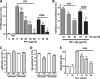
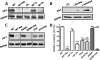
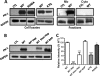
The serpin protease nexin-1 (PN-1) is expressed by vascular cells and secreted by platelets upon activation, and it is known to interact with several modulators of angiogenesis, such as proteases, matrix proteins, and glycosaminoglycans. We therefore investigated the impact of PN-1 on endothelial cell angiogenic responses in vitro and ex vivo and in vivo in PN-1-deficient mice. We found that PN-1 is antiangiogenic in vitro: it inhibited vascular endothelial growth factor (VEGF)-induced endothelial cell responses, including proliferation, migration, and capillary tube formation, and decreased cell spreading on vitronectin. These effects do not require the antiprotease activity of PN-1 but involve PN-1 binding to glycosaminoglycans. In addition, our results indicated that PN-1 does not act by blocking VEGF binding to its heparan sulfate proteoglycan coreceptors. The results obtained in vitro were supported ex vivo in PN-1-deficient mice, where the microvascular network sprouting from aortic rings was significantly enhanced. Moreover, in vivo, neovessel formation was promoted in the Matrigel plug assay in PN-1-deficient mice compared to wild-type mice, and these effects were reversed by the addition of recombinant PN-1. Taken together, our results demonstrate that PN-1 has direct antiangiogenic properties and is a yet-unrecognized player in the angiogenic balance.
Figures


References
-
- Asanuma K, et al. 2007. Protein C inhibitor inhibits breast cancer cell growth, metastasis and angiogenesis independently of its protease inhibitory activity. Int. J. Cancer 121: 955–965 - PubMed
-
- Baker JB, Low DA, Simmer RL, Cunningham DD. 1980. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell 21: 37–45 - PubMed
-
- Boulaftali Y, et al. 2010. Anticoagulant and antithrombotic properties of platelet protease nexin-1. Blood 115: 97–106 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
