Laser captured hepatocytes show association of butyrylcholinesterase gene loss and fibrosis progression in hepatitis C-infected drug users
- PMID: 22331678
- PMCID: PMC3388175
- DOI: 10.1002/hep.25655
Laser captured hepatocytes show association of butyrylcholinesterase gene loss and fibrosis progression in hepatitis C-infected drug users
Abstract
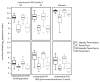
Chronic hepatitis C virus (HCV) infection is complicated by hepatic fibrosis. Hypothesizing that early fibrogenic signals may originate in cells susceptible to HCV infection, hepatocyte gene expression was analyzed from persons with chronic HCV at different stages of liver fibrosis. Four HCV-infected subjects with precirrhosis liver fibrosis (Ishak fibrosis 3-5) were matched for age, race, and gender to five HCV-infected subjects with no evidence of fibrosis (Ishak fibrosis 0). Hepatocytes from each subject were isolated from liver biopsies using laser capture microdissection. Transcriptome profiling was performed on hepatocyte RNA using hybridization arrays. We found that hepatocytes in precirrhosis fibrosis were depleted for genes involved in small molecule and drug metabolism, especially butyrylcholinesterase (BCHE), a gene involved in the metabolism of drugs of abuse. Differential expression of BCHE was validated in the same tissues and cross-sectionally in an expanded cohort of 143 HCV-infected individuals. In a longitudinal study, serum BCHE activity was already decreased at study inception in 19 fibrosis progressors compared with 20 fibrosis nonprogressors (P < 0.05). Nonprogressors also had decreased BCHE activity over time compared with initial values, but these evolved a median (range) 8.6 (7.8-11.4) years after the study period inception (P < 0.05). Laser captured portal tracts were enriched for immune related genes when compared with hepatocytes but precirrhosis livers lost this enrichment.
Conclusion: Chronic HCV is associated with hepatocyte BCHE loss years before hepatic synthetic function is impaired. These results indicate that BCHE may be involved in the pathogenesis of HCV-related fibrosis among injection drug users.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures




References
-
- World Health Organization. Hepatitis C: global prevalence. Weekly Epidemiological Record. 1997;72:341–348.
-
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006 May 16;144(10):705–714. - PubMed
-
- Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005 Aug;9(3):383–98. vi. - PubMed
-
- Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000 Jan 28;275(4):2247–2250. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
