N-acetyl serotonin derivatives as potent neuroprotectants for retinas
- PMID: 22331903
- PMCID: PMC3295250
- DOI: 10.1073/pnas.1119201109
N-acetyl serotonin derivatives as potent neuroprotectants for retinas
Abstract
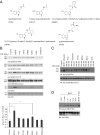
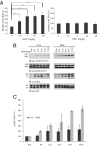
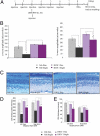
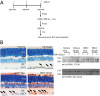
N-acetylserotonin (NAS) is synthesized from serotonin by arylalkylamine N-acetyltransferase (AANAT), which is predominantly expressed in the pineal gland and retina. NAS activates TrkB in a circadian manner and exhibits antidepressant effects in a TrkB-dependent manner. It also enhances neurogenesis in hippocampus in sleep-deprived mice. Here we report the identification of NAS derivatives that possess much more robust neurotrophic effects with improved pharmacokinetic profiles. The compound N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-2-oxopiperidine-3-carboxamide (HIOC) selectively activates TrkB receptor with greater potency than NAS. It potently protects retinas from light-induced retinal degeneration (LIRD), which is tightly coupled with pronounced TrkB activation in retinas. Pharmacokinetic studies demonstrate that this compound is stable in serum and liver microsomes. It can pass the blood-brain barrier and blood-retinal barrier. Hence, HIOC is a good lead compound for further drug development for treating retinal degenerative diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

