High-grade serous ovarian cancer arises from fallopian tube in a mouse model
- PMID: 22331912
- PMCID: PMC3309733
- DOI: 10.1073/pnas.1117135109
High-grade serous ovarian cancer arises from fallopian tube in a mouse model
Abstract
Although ovarian cancer is the most lethal gynecologic malignancy in women, little is known about how the cancer initiates and metastasizes. In the last decade, new evidence has challenged the dogma that the ovary is the main source of this cancer. The fallopian tube has been proposed instead as the primary origin of high-grade serous ovarian cancer, the subtype causing 70% of ovarian cancer deaths. By conditionally deleting Dicer, an essential gene for microRNA synthesis, and Pten, a key negative regulator of the PI3K pathway, we show that high-grade serous carcinomas arise from the fallopian tube in mice. In these Dicer-Pten double-knockout mice, primary fallopian tube tumors spread to engulf the ovary and then aggressively metastasize throughout the abdominal cavity, causing ascites and killing 100% of the mice by 13 mo. Besides the clinical resemblance to human serous cancers, these fallopian tube cancers highly express genes that are known to be up-regulated in human serous ovarian cancers, also demonstrating molecular similarities. Although ovariectomized mice continue to develop high-grade serous cancers, removal of the fallopian tube at an early age prevents cancer formation--confirming the fallopian tube origin of the cancer. Intriguingly, the primary carcinomas are first observed in the stroma of the fallopian tube, suggesting that these epithelial cancers have a mesenchymal origin. Thus, this mouse model demonstrates a paradigm for the origin and initiation of high-grade serous ovarian carcinomas, the most common and deadliest ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
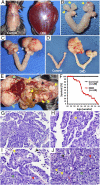

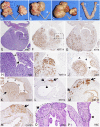
Comment in
-
Nonovarian origins of ovarian cancer.Proc Natl Acad Sci U S A. 2012 Mar 6;109(10):3608-9. doi: 10.1073/pnas.1201029109. Epub 2012 Feb 22. Proc Natl Acad Sci U S A. 2012. PMID: 22357760 Free PMC article. No abstract available.
References
-
- Koonings PP, Campbell K, Mishell DR, Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: A 10-year review. Obstet Gynecol. 1989;74:921–926. - PubMed
-
- Seidman JD, et al. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–44. - PubMed
-
- Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Bukowski RM, Ozols RF, Markman M. The management of recurrent ovarian cancer. Semin Oncol. 2007;34(Suppl 2):S1–S15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

