Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer
- PMID: 22331951
- PMCID: PMC3341106
- DOI: 10.1200/JCO.2011.38.0261
Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer
Abstract
Purpose: Aromatase inhibitors (AIs) are effective for treatment of hormone receptor-positive breast cancer, but adherence and persistence with therapy are poor. Predictors of treatment discontinuation are not clearly defined. It is unknown whether patients with intolerable toxicity from one AI are able to tolerate another.
Patients and methods: Women with early-stage breast cancer initiating AI therapy were enrolled onto a multicenter, prospective, open-label randomized trial of exemestane versus letrozole. Patients completed symptom questionnaires at baseline and serially during therapy. Patients who developed AI-associated intolerable symptoms and discontinued treatment were given the option to switch to the other study AI after a 2- to 8-week washout period.
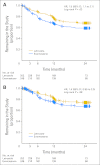
Results: Of the 503 enrolled women, 32.4% discontinued initial AI therapy within 2 years because of adverse effects; 24.3% discontinued specifically because of musculoskeletal symptoms. Median time to treatment discontinuation as a result of any symptom was 6.1 months (range, 0.1 to 21.2 months) and was significantly shorter in patients randomly assigned to exemestane (hazard ratio [HR], 1.5; 95% CI, 1.1 to 2.1; P = .02). Younger age and taxane-based chemotherapy were associated with higher likelihood of treatment discontinuation (HR, 1.4; 95% CI, 1.02 to 1.9; P = .04; and HR, 1.9; 95% CI, 1.00 to 3.6; P = .048, respectively). Of the 83 patients who chose to switch to the second AI, 38.6% continued the alternate AI for a median of 13.7 months.
Conclusion: Premature discontinuation of initial AI therapy as a result of symptoms is common, although more than one third of patients may be able to tolerate a different AI medication. Additional research is needed to identify predictive tools and interventions for AI-associated treatment-emergent symptoms.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. - PubMed
-
- Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol. 2008;26:1948–1955. - PubMed
-
- Goss PE, Ingle JN, Chapman J-AW, et al. Final analysis of NCIC CTG MA.27: A randomized phase III trial of exemestane versus anastrozole in postmenopausal women with hormone receptor positive primary breast cancer. 33rd Annual San Antonio Breast Cancer Symposium; December 8-12, 2010; San Antonio, TX. abstr S1-1.
-
- Forbes JF, Cuzick J, Buzdar A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

