Pharmacokinetics of oral taurine in healthy volunteers
- PMID: 22331997
- PMCID: PMC3275936
- DOI: 10.4061/2010/346237
Pharmacokinetics of oral taurine in healthy volunteers
Abstract
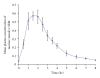
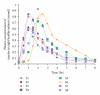
Taurine, a sulfur-containing amino acid, is a normal constituent of the human diet. Little is known of the pharmacokinetics of taurine in man after oral administration. We studied the pharmacokinetics of 4 g taurine in eight healthy male volunteers (median age 27.5, range 22-45) following orally administration in the fasting state in the morning. Blood samples were taken at regular intervals and plasma taurine concentration was measured by a modified HPLC method. Data were subjected to noncompartmental analysis. Maximum plasma taurine concentration (C(max)) was measured at 1.5 ± 0.6 hr after administration as 86.1 ± 19.0 mg/L (0.69 ± 0.15 mmol). Plasma elimination half-life (T(1/2)) and the ratio of clearance/bioavailability (Cl/F) were 1.0 ± 0.3 hr and 21.1 ± 7.8 L/hr, respectively. Since taurine is occasionally used in therapeutics as a medicine, the pharmacokinetics and effects of oral taurine in healthy volunteers would be useful in the future studies of taurine in pharmacology and nutrition.
Figures
References
-
- Huxtable R, Sebring L. Cardiovascular actions of taurine. In: Kuriyama K, Huxtable R, Iwata H, editors. Sulfur Amino Acids Biochemical Aspects. New York, NY, USA: Alan R. Liss; 1983. pp. 5–37.
-
- Jacobsen JG, Smith LH. Biochemistry and physiology of taurine and taurine derivatives. Physiological Reviews. 1968;48(2):424–511. - PubMed
-
- Waterfield CJ, Turton JA, Scales MD, Timbrell JA. Taurine, a possible urinary marker of liver damage: a study of taurine excretion in carbon tetrachloride-treated rats. Archives of Toxicology. 1991;65(7):548–555. - PubMed
-
- Obinata K, Maruyama T, Hayashi M, Watanabe T, Nittono H. Effect of taurine on the fatty liver of children with simple obesity. Advances in Experimental Medicine and Biology. 1996;403:607–613. - PubMed
-
- Yamauchi-Takihara K, Azuma J, Kishimoto S. Taurine protection against experimental arterial calcinosis in mice. Biochemical and Biophysical Research Communications. 1986;140(2):679–683. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous