Formation of the 42-mer Amyloid β Radical and the Therapeutic Role of Superoxide Dismutase in Alzheimer's Disease
- PMID: 22332002
- PMCID: PMC3276080
- DOI: 10.4061/2011/654207
Formation of the 42-mer Amyloid β Radical and the Therapeutic Role of Superoxide Dismutase in Alzheimer's Disease
Abstract
Oxidative stress is closely involved in age-related diseases and ageing itself. There is evidence of the leading contribution of oxidative damage to neurodegenerative disease, in contrast to other diseases where oxidative stress plays a secondary role. The 42-mer amyloid β (Aβ42) peptide is thought to be a culprit in the pathogenesis of Alzheimer's disease (AD). Aβ42 aggregates form the oligomeric assembly and show neurotoxicity, causing synaptic dysfunction. Aβ42 also induces tissue oxidation (DNA/RNA, proteins, and lipids) through trace metals (Cu, Zn, and Fe), which can be protected by antioxidant enzymes, vitamin C, and vitamin E. Superoxide dismutase catalyzes the conversion of toxic superoxide radical to less reactive hydrogen peroxide, contributing to protection from AD. Here we review the involvement of oxidative stress in AD progression induced from an imbalance between the radical formation of Aβ42 itself together with unique turn structure at positions Glu22 and Asp23 and several defense systems.
Figures
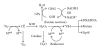

References
-
- Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11(3):298–300. - PubMed
-
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nature Medicine. 2004;10:S18–S25. - PubMed
-
- Marlatt M, Lee HG, Perry G, Smith MA, Zhu X. Sources and mechanisms of cytoplasmic oxidative damage in Alzheimer’s disease. Acta Neurobiologiae Experimentalis. 2004;64(1):81–87. - PubMed
-
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiological Reviews. 1979;59(3):527–605. - PubMed
-
- Wallace DC, Melov S. Radicals r’aging. Nature Genetics. 1998;19(2):105–106. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

