Imaging of Childhood Interstitial Lung Disease
- PMID: 22332031
- PMCID: PMC3269227
- DOI: 10.1089/ped.2010.0010
Imaging of Childhood Interstitial Lung Disease
Abstract
The aphorism that children are not little adults certainly applies for the imaging of interstitial lung disease. Acquiring motion-free images of fine pulmonary structures at desired lung volumes is much more difficult in children than in adults. Several forms of interstitial lung disease are unique to children, and some forms of interstitial lung disease encountered in adults rarely, if ever, occur in children. Meticulous attention to imaging technique and specialized knowledge are required to properly perform and interpret chest imaging studies obtained for the evaluation of childhood interstitial lung disease (chILD). This review will address technique recommendations for imaging chILD, the salient imaging findings in various forms of chILD, and the efficacy of imaging in the diagnosis and management of chILD.
Figures
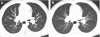
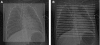
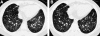

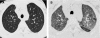
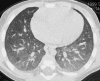
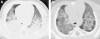

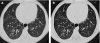


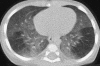
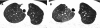
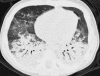
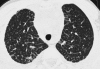
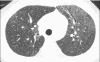
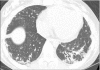
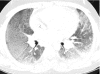
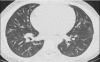


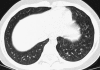
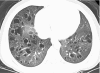
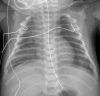

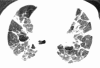
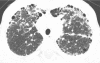
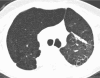
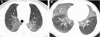
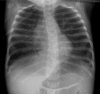
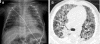

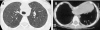



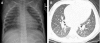

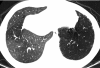



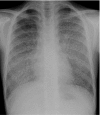
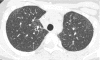

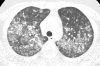

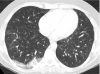


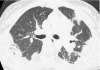

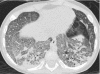
References
-
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
-
- Deutsch GH. Young LR. Deterding RR. Fan LL. Dell SD. Bean JA. Brody AS. Nogee LM. Trapnell BC. Langston C. Albright EA. Askin FB. Baker P. Chou PM. Cool CM. Coventry SC. Cutz E. Davis MM. Dishop MK. Galambos C. Patterson K. Travis WD. Wert SE. White FV Pathology Cooperative Group; ChILD Research Co-operative. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–1128. - PMC - PubMed
-
- Clement A ERS Task Force. Task force on chronic interstitial lung disease in immunocompetent children. Eur Respir J. 2004;24:686–697. - PubMed
-
- Lynch DA. Brasch RC. Hardy KA. Webb WR. Pediatric pulmonary disease: assessment with high-resolution ultrafast CT. Radiology. 1990;176:243–248. - PubMed
-
- Brody AS. Imaging considerations: interstitial lung disease in children. Radiol Clin North Am. 2005;43:391–403. - PubMed
LinkOut - more resources
Full Text Sources

