Enhanced tumor treatment using biofunctional indocyanine green-containing nanostructure by intratumoral or intravenous injection
- PMID: 22332810
- PMCID: PMC3418867
- DOI: 10.1021/mp200526m
Enhanced tumor treatment using biofunctional indocyanine green-containing nanostructure by intratumoral or intravenous injection
Abstract
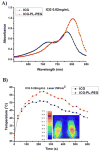
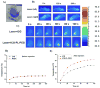
Indocyanine green (ICG) is a conventional dye that can be used in clinical near-infrared (NIR) imaging, and it is also an effective light absorber for laser-mediated photothermal therapy. However, applications of ICG were limited due to its fast degradation in aqueous media and quick clearance from the body. Herein, an ICG-containing nanostructure, ICG-PL-PEG, was developed for photothermal therapy, which was self-assembled by ICG and phospholipid-polyethylene glycol (PL-PEG). Our in vitro and in vivo experiments demonstrated that ICG-PL-PEG suspension was more efficient in producing a NIR-dependent temperature increase than ICG alone, due to the increase of ICG monomers from the addition of PL-PEG to match the central wavelength of the 808 nm laser. When conjugated with integrin α(v)β(3) monoclonal antibody (mAb), ICG-PL-PEG could be selectively internalized and retained in target tumor cells. Irradiation of an 808 nm laser after intravenous administration of ICG-PL-PEG-mAb resulted in tumor suppression in mice, while ICG alone had only limited effect. This is the first time an ICG-containing nanostructure has been used through systemic administration to achieve an efficient in vivo photothermal effect for cancer treatment. Therefore, ICG-PL-PEG could be used as a fluorescent marker as well as a light-absorber for imaging-guided photothermal therapy. All the components of ICG-PL-PEG have been approved for human use. Therefore, this unique ICG-containing nanostructure has great potential in clinical applications.
Figures



References
-
- Nolsoe CP, Torp-Pedersen S, Burcharth F, Horn T, Pedersen S, Christensen NE, Olldag ES, Andersen PH, Karstrup S, Lorentzen T, et al. Interstitial hyperthermia of colorectal liver metastases with a US-guided Nd-YAG laser with a diffuser tip: a pilot clinical study. Radiology. 1993;187:333–337. - PubMed
-
- Amin Z, Donald JJ, Masters A, Kant R, Steger AC, Bown SG, Lees WR. Hepatic metastases: interstitial laser photocoagulation with real-time US monitoring and dynamic CT evaluation of treatment. Radiology. 1993;187:339–347. - PubMed
-
- Anghileri LJ, Robert J. Hyperthermia in Cancer Treatment. CRC Press; Boca Raton FL: 1986. pp. 59–78.
-
- Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

