From Drosophila to humans: reflections on the roles of the prolyl isomerases and chaperones, cyclophilins, in cell function and disease
- PMID: 22332926
- PMCID: PMC3668307
- DOI: 10.3109/01677063.2011.647143
From Drosophila to humans: reflections on the roles of the prolyl isomerases and chaperones, cyclophilins, in cell function and disease
Abstract
Despite remarkable advances in human genetics and other genetic model systems, the fruit fly, Drosophila melanogaster, remains a powerful experimental tool to probe with ease the inner workings of a myriad of biological and pathological processes, even when evolutionary forces impart apparent divergences to some of such processes. The understanding of such evolutionary differences provides mechanistic insights into genotype-phenotype correlations underpinning biological processes across metazoans. The pioneering work developed by the William Pak laboratory for the past four decades, and the work of others, epitomize the notion of how the Drosophila system breaks new fertile ground or complements research fields of high scientific and medical relevance. Among the three major genetic complementation groups produced by the Pak's laboratory and impairing distinct facets of photoreceptor neuronal function, the nina group (ninaA, …., ninaJ) selectively affects the biogenesis of G protein-coupled receptors (GPCRs), mediating the photoconversion and transduction of light stimuli. Among the nina genes identified, ninaA arguably assumes heightened significance for several reasons. First, it presents unique physiological selectivity toward the biogenesis of a subset of GPCRs, a standalone biological manifestation yet to be discerned for most mammalian homologues of NinaA. Second, NinaA belongs to a family of proteins, immunophilins, which are the primary targets for immunosuppressive drugs at the therapeutic forefront of a multitude of medical conditions. Third, NinaA closest homologue, cyclophilin B (CyPB/PPIB), is an immunophilin whose loss-of-function was found recently to cause osteogenesis imperfecta in the human. This report highlights advances made by studies on some members of immunophilins, the cyclophilins. Finally, it reexamines critically data and dogmas derived from past and recent genetic, structural, biological, and pathological studies on NinaA and few other cyclophilins that support some of such paradigms to be less than definite and advance our understanding of the roles of cyclophilins in cell function, disease, and therapeutic interventions.
Figures
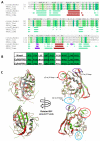
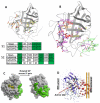
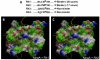
References
-
- Abagyan R, Orry A, Raush E, Totrov M. ICM User Guide 3.7. La Jolla, CA; Molsoft LLC: 2010.
-
- Abagyan R, Totrov M. Biased probability Monte Carlo conformational searches and electrostatic calculations for peptides and proteins. J Mol Biol. 1994;235:983–1002. - PubMed
-
- Abagyan R, Totrov M, Kuznetsov D. ICM-a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem. 1994;15:488–506.
-
- Abagyan RA, Batalov S. Do aligned sequences share the same fold? J Mol Biol. 1997;273:355–368. - PubMed
-
- Allain F, Vanpouille C, Carpentier M, Slomianny MC, Durieux S, Spik G. Interaction with glycosaminoglycans is required for cyclophilin B to trigger integrin-mediated adhesion of peripheral blood T lymphocytes to extracellular matrix. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2714–2719. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
