A phase I trial of pan-Bcl-2 antagonist obatoclax administered as a 3-h or a 24-h infusion in combination with carboplatin and etoposide in patients with extensive-stage small cell lung cancer
- PMID: 22333598
- PMCID: PMC3305978
- DOI: 10.1038/bjc.2012.21
A phase I trial of pan-Bcl-2 antagonist obatoclax administered as a 3-h or a 24-h infusion in combination with carboplatin and etoposide in patients with extensive-stage small cell lung cancer
Abstract
Background: Bcl-2 family genes are frequently amplified in small cell lung cancer (SCLC). A phase I trial was conducted to evaluate the safety of obatoclax, a Bcl-2 family inhibitor, given in combination with standard chemotherapy.
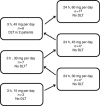
Methods: Eligible patients (3-6 per cohort) had extensive-stage SCLC, measurable disease, ≤ 1 before therapy, Eastern Cooperative Oncology Group performance status 0 or 1, and adequate organ function. Patients were treated with escalating doses of obatoclax, either as a 3- or 24-h infusion, on days 1-3 of a 21-day cycle, in combination with carboplatin (area under the curve 5, day 1 only) and etoposide (100 mg m(-2), days 1-3). The primary endpoint was to determine the maximum tolerated dose of obatoclax.
Results: Twenty-five patients (56% male; median age 66 years) were enrolled in three dose cohorts for each schedule. Maximum tolerated dose was established with the 3-h infusion at 30 mg per day and was not reached with the 24-h infusion. Compared with the 24-h cohorts, the 3-h cohorts had higher incidence of central nervous system (CNS) adverse events (AEs); dose-limiting toxicities were somnolence, euphoria, and disorientation. These CNS AEs were transient, resolving shortly after the end of infusion, and without sequelae. The response rate was 81% in the 3-h and 44% in the 24-h infusion cohorts.
Conclusion: Although associated with a higher incidence of transient CNS AEs than the 24-h infusion, 3-h obatoclax infusion combined with carboplatin-etoposide was generally well tolerated at doses of 30 mg per day. Though patient numbers were small, there was a suggestion of improved efficacy in the 3-h infusion group. Obatoclax 30 mg infused intravenously over 3 h on 3 consecutive days will be utilised in future SCLC studies.
Figures
References
-
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho YJ, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, Maher E, Kaye FJ, Sasaki H, Tepper JE, Fletcher JA, Tabernero J, Baselga J, Tsao MS, Demichelis F, Rubin MA, Janne PA, Daly MJ, Nucera C, Levine RL, Ebert BL, Gabriel S, Rustgi AK, Antonescu CR, Ladanyi M, Letai A, Garraway LA, Loda M, Beer DG, True LD, Okamoto A, Pomeroy SL, Singer S, Golub TR, Lander ES, Getz G, Sellers WR, Meyerson M (2010) The landscape of somatic copy-number alteration across human cancers. Nature 463: 899–905 - PMC - PubMed
-
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, Hann CL, McKeegan EM, Litvinovich E, Hemken PM, Dive C, Enschede SH, Nolan C, Chiu YL, Busman T, Xiong H, Krivoshik AP, Humerickhouse R, Shapiro GI, Rudin CM (2011) Phase 1 study of navitoclax (ABT-263), a novel BCL-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol 29: 909–916 - PMC - PubMed
-
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 24: 4539–4544 - PubMed

