Largazole: from discovery to broad-spectrum therapy
- PMID: 22334030
- PMCID: PMC4777309
- DOI: 10.1039/c2np00066k
Largazole: from discovery to broad-spectrum therapy
Abstract
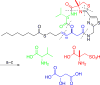
The cyclic depsipeptide largazole from a cyanobacterium of the genus Symploca is a marine natural product with a novel chemical scaffold and potently inhibits class I histone deacetylases (HDACs). Largazole possesses highly differential growth-inhibitory activity, preferentially targeting transformed over non-transformed cells. The intriguing structure and biological activity of largazole have attracted strong interest from the synthetic chemistry community to establish synthetic routes to largazole and to investigate its potential as a cancer therapeutic. This Highlight surveys recent advances in this area with a focus on the discovery, synthesis, target identification, structure-activity relationships, HDAC8-largazole thiol crystal structure, and biological studies, including in vivo anticancer and osteogenic activities.
Figures





References
-
- Moore RE, Corbett TH, Patterson GML, Valeriote FA. Curr Pharm Des. 1996;2:317–330.
-
- Liang J, Moore RE, Moher ED, Munroe JE, Al-awar RS, Hay DA, Varie DL, Zhang TY, Aikins JA, Martinelli MJ, Shih C, Ray JE, Gibson LL, Vasudevan V, Polin L, White K, Kushner J, Simpson C, Pugh S, Corbett TH. Invest New Drugs. 2005;23:213–224. - PubMed
-
- Luesch H, Moore RE, Paul VJ, Mooberry SL, Corbett TH. J Nat Prod. 2001;64:907–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

