Luminescent di- and polynuclear organometallic gold(I)-metal (Au2, {Au2Ag}n and {Au2Cu}n) compounds containing bidentate phosphanes as active antimicrobial agents
- PMID: 22334444
- PMCID: PMC3371653
- DOI: 10.1002/chem.201103145
Luminescent di- and polynuclear organometallic gold(I)-metal (Au2, {Au2Ag}n and {Au2Cu}n) compounds containing bidentate phosphanes as active antimicrobial agents
Abstract
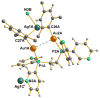
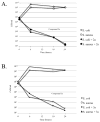
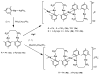
The reaction of new dinuclear gold(I) organometallic complexes containing mesityl ligands and bridging bidentate phosphanes [Au(2)(mes)(2)(μ-LL)] (LL=dppe: 1,2-bis(diphenylphosphano)ethane 1a, and water-soluble dppy: 1,2-bis(di-3-pyridylphosphano)ethane 1b) with Ag(+) and Cu(+) lead to the formation of a family of heterometallic clusters with mesityl bridging ligands of the general formula [Au(2)M(μ-mes)(2) (μ-LL)][A] (M=Ag, A=ClO(4)(-), LL=dppe 2a, dppy 2b; M=Ag, A=SO(3)CF(3)(-), LL=dppe 3a, dppy 3b; M=Cu, A=PF(6)(-), LL=dppe 4a, dppy 4b). The new compounds were characterized by different spectroscopic techniques and mass spectrometry The crystal structures of [Au(2)(mes)(2)(μ-dppy)] (1b) and [Au(2)Ag(μ-mes)(2)(μ-dppe)][SO(3)CF(3)] (3a) were determined by a single-crystal X-ray diffraction study. 3a in solid state is not a cyclic trinuclear Au(2)Ag derivative but it gives an open polymeric structure instead, with the {Au(2)(μ-dppe)} fragments "linked" by {Ag(μ-mes)(2)} units. The very short distances of 2.7559(6) Å (Au-Ag) and 2.9229(8) Å (Au-Au) are indicative of gold-silver (metallophilic) and aurophilic interactions. A systematic study of their luminescence properties revealed that all compounds are brightly luminescent in solid state, at room temperature (RT) and at 77 K, or in frozen DMSO solutions with lifetimes in the microsecond range and probably due to the self-aggregation of [Au(2)M(μ-mes)(2)(μ-LL)](+) units (M=Ag or Cu; LL=dppe or dppy) into an extended chain structure, through Au-Au and/or Au-M metallophilic interactions, as that observed for 3a. In solid state the heterometallic Au(2)M complexes with dppe (2a-4a) show a shift of emission maxima (from ca. 430 to the range of 520-540 nm) as compared to the parent dinuclear organometallic product 1a while the complexes with dppy (2b-4b) display a more moderate shift (505 for 1b to a max of 563 nm for 4b). More importantly, compound [Au(2)Ag(μ-mes)(2)(μ-dppy)]ClO(4) (2b) resulted luminescent in diluted DMSO solution at room temperature. Previously reported compound [Au(2)Cl(2)(μ-LL)] (LL dppy 5b) was also studied for comparative purposes. The antimicrobial activity of 1-5 and Ag[A] (A=ClO(4)(-), SO(3)CF(3)(-)) against gram-positive and gram-negative bacteria and yeast was evaluated. Most tested compounds displayed moderate to high antibacterial activity while heteronuclear Au(2)M derivatives with dppe (2a-4a) were the more active (minimum inhibitory concentration 10 to 1 μg mL(-1)). Compounds containing silver were ten times more active to gram-negative bacteria than the parent dinuclear compound 1a or silver salts. Au(2)Ag compounds with dppy (2b, 3b) were also potent against fungi.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
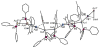



Similar articles
-
Metallophilic bonding and agostic interactions in gold(I) and silver(I) complexes bearing a thiotetrazole unit.Inorg Chem. 2011 Mar 21;50(6):2675-84. doi: 10.1021/ic102595d. Epub 2011 Feb 10. Inorg Chem. 2011. PMID: 21309537
-
Syntheses, Structures, and Antimicrobial Activities of Gold(I)- and Copper(I)- N-Heterocyclic Carbene (NHC) Complexes Derived from Basket-Shaped Dinuclear Ag(I)-NHC Complex.Inorg Chem. 2018 Sep 17;57(18):11322-11332. doi: 10.1021/acs.inorgchem.8b00011. Epub 2018 Aug 31. Inorg Chem. 2018. PMID: 30168718
-
Synthesis and structures of group 11 metal triazenide complexes: ligand supported metallophilic interactions.Inorg Chem. 2009 Sep 7;48(17):8613-22. doi: 10.1021/ic901051f. Inorg Chem. 2009. PMID: 19637866
-
Silver and Gold Containing Compounds of p-Block Elements As Perspective Materials for UV Plasmonics.ACS Omega. 2023 Apr 12;8(16):14321-14341. doi: 10.1021/acsomega.2c05943. eCollection 2023 Apr 25. ACS Omega. 2023. PMID: 37125114 Free PMC article. Review.
-
Phosphane-based cyclodextrins as mass transfer agents and ligands for aqueous organometallic catalysis.Molecules. 2012 Nov 2;17(11):13062-72. doi: 10.3390/molecules171113062. Molecules. 2012. PMID: 23124474 Free PMC article. Review.
Cited by
-
Auranofin and related heterometallic gold(I)-thiolates as potent inhibitors of methicillin-resistant Staphylococcus aureus bacterial strains.J Inorg Biochem. 2014 Sep;138:81-88. doi: 10.1016/j.jinorgbio.2014.05.008. Epub 2014 May 28. J Inorg Biochem. 2014. PMID: 24935090 Free PMC article.
-
Gold-Derived Molecules as New Antimicrobial Agents.Front Microbiol. 2022 Mar 23;13:846959. doi: 10.3389/fmicb.2022.846959. eCollection 2022. Front Microbiol. 2022. PMID: 35401486 Free PMC article. Review.
-
Functional utility of gold complexes with phosphorus donor ligands in biological systems.Coord Chem Rev. 2025 Jan 1;522:216208. doi: 10.1016/j.ccr.2024.216208. Epub 2024 Sep 27. Coord Chem Rev. 2025. PMID: 39552640
-
Regulation of the aggregation behavior of human islet amyloid polypeptide fragment by titanocene complexes.J Biol Inorg Chem. 2017 Oct;22(7):1065-1074. doi: 10.1007/s00775-017-1484-x. Epub 2017 Aug 11. J Biol Inorg Chem. 2017. PMID: 28801867
-
Digold(I) Thianthrenyl Complexes. Effect of Diphosphine Ligands on Molecular Structures in the Solid State and in Solution.ACS Omega. 2022 Mar 11;7(11):9594-9601. doi: 10.1021/acsomega.1c06938. eCollection 2022 Mar 22. ACS Omega. 2022. PMID: 35350371 Free PMC article.
References
-
- Yam VWW, Cheng ECC. Chem Soc Rev. 2008;37:1806–1813. - PubMed
- Barbieri A, Accorsi G, Aramaroli N. Chem Commun. 2008:2185–2193. - PubMed
- Fernández EJ, Laguna A, López-de-Luzuriaga JM. Dalton Trans. 2007:1969–1981. - PubMed
- Vogler A, Kunkely H. Coord Chem Rev. 2001;219–221:489–507.
- Schmidbaur H. Chem Soc Rev. 1995;24:391–400.
-
- Pyykko P, Zhao Y. Angew Chem Int Ed Engl. 1991;30:604–605.
-
- King C, Wang J-C, Khan MNI, Fackler JP., Jr Inorg Chem. 1989;28:2145–2149.
-
- Che C-M, Kwong HL, Yam VW-W, Cho KC. J Chem Soc, Chem Commun. 1989:885–886.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

