Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: meta-epidemiological study
- PMID: 22334559
- PMCID: PMC3279328
- DOI: 10.1136/bmj.e813
Impact of single centre status on estimates of intervention effects in trials with continuous outcomes: meta-epidemiological study
Abstract
Objective: To compare estimates of intervention effects between single centre and multicentre randomised controlled trials with continuous outcomes.
Design: Meta-epidemiological study.
Data sources: 26 meta-analyses totalling 292 randomised controlled trials (177 single centre, 115 multicentre) with continuous outcomes published between January 2007 and January 2010 in the Cochrane database of systematic reviews.
Data extraction: Data were extracted on characteristics of trials, single or multicentre status, risk of bias using the risk of bias tool of the Cochrane Collaboration, and results.
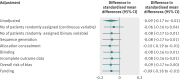
Data synthesis: The intervention effects were estimated with standardised mean differences. For each meta-analysis, random effects meta-regression was used to estimate the difference in standardised mean differences between single centre and multicentre trials. Differences in standardised mean differences were then pooled across meta-analyses by a random-effects meta-analysis model. A combined difference in standardised mean differences of less than 0 indicated that single centre trials showed larger treatment effects, on average, than did multicentre trials. Because single centre trials may be more prone to publication bias and may have lower methodological quality than multicentre trials, sensitivity analyses were done with adjustment for sample size and domains of the risk of bias tool.
Results: Single centre trials showed larger intervention effects than did multicentre trials (combined difference in standardised mean differences -0.09, 95% confidence interval -0.17 to -0.01, P=0.04), with low heterogeneity across individual meta-analyses (I(2)=0%, between meta-analyses variance τ(2)=0.00). Adjustment for sample size slightly attenuated the difference (-0.08, -0.17 to 0.01). Adjustment for risk of bias yielded similar estimates with wider confidence intervals, some of them crossing 0 (-0.09, -0.17 to 0.00 for overall risk of bias).
Conclusions: On average, single centre clinical trials with continuous outcomes showed slightly larger intervention effects than did multicentre trials. Further research is needed to investigate potential causes of these differences.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures


References
-
- Nuesch E, Reichenbach S, Trelle S, Rutjes AW, Liewald K, Sterchi R, et al. The importance of allocation concealment and patient blinding in osteoarthritis trials: a meta-epidemiologic study. Arthritis Rheum 2009;61:1633-41. - PubMed
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
