Primary alveolar epithelial cell surface membrane microdomain function is required for Pneumocystis β-glucan-induced inflammatory responses
- PMID: 22334619
- PMCID: PMC3645441
- DOI: 10.1177/1753425912436763
Primary alveolar epithelial cell surface membrane microdomain function is required for Pneumocystis β-glucan-induced inflammatory responses
Abstract
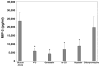
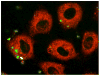
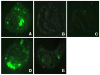
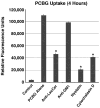
Intense lung inflammation characterizes respiratory failure associated with Pneumocystis pneumonia. Our laboratory has previously demonstrated that alveolar epithelial cells (AECs) elaborate inflammatory cytokines and chemokines in response to the Pneumocystis carinii cell wall constituent β-(1→3)-glucan (PCBG), and that these responses require lactosylceramide, a prominent glycosphingolipid constituent of certain cell membrane microdomains. The relevance of membrane microdomains, also termed plasma membrane lipid rafts, in cell signaling and macromolecule handling has been increasingly recognized in many biologic systems, but their role in P. carinii-induced inflammation is unknown. To investigate the mechanisms of microdomain-dependent P. carinii-induced inflammation, we challenged primary rat AECs with PCBG with or without pre-incubation with inhibitors of microdomain function. Glycosphingolipid and cholesterol rich microdomain inhibition resulted in significant attenuation of P. carinii-induced expression of TNF-α and the rodent C-X-C chemokine MIP-2, as well as their known inflammatory secondary signaling pathways. We have previously shown that protein kinase C (PKC) is activated by PCBG challenge and herein show that PKC localizes to AEC microdomains. We also demonstrate by conventional microscopy, fluorescence microscopy, confocal microscopy and spectrophotofluorimetry that AECs internalize fluorescently-labeled PCBG by microdomain-mediated mechanisms, and that anti-microdomain pretreatments prevent internalization. Taken together, these data suggest an important role for AEC microdomain function in PCBG-induced inflammatory responses. This offers a potential novel target for therapeutics for a condition that continues to exert unacceptable morbidity and mortality among immunocompromised populations.
Figures

Similar articles
-
Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms.Am J Respir Cell Mol Biol. 2005 Jun;32(6):490-7. doi: 10.1165/rcmb.2004-0300OC. Epub 2005 Mar 3. Am J Respir Cell Mol Biol. 2005. PMID: 15746433 Free PMC article.
-
Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism.J Biol Chem. 2003 Jan 17;278(3):2043-50. doi: 10.1074/jbc.M209715200. Epub 2002 Nov 4. J Biol Chem. 2003. PMID: 12419803
-
Pneumocystis stimulates MCP-1 production by alveolar epithelial cells through a JNK-dependent mechanism.Am J Physiol Lung Cell Mol Physiol. 2007 Jun;292(6):L1495-505. doi: 10.1152/ajplung.00452.2006. Epub 2007 Feb 16. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17307812
-
Involvement of glycosphingolipid-enriched lipid rafts in inflammatory responses.Front Biosci (Landmark Ed). 2015 Jan 1;20(2):325-34. doi: 10.2741/4312. Front Biosci (Landmark Ed). 2015. PMID: 25553454 Review.
-
Significance of glycosphingolipid fatty acid chain length on membrane microdomain-mediated signal transduction.FEBS Lett. 2010 May 3;584(9):1642-52. doi: 10.1016/j.febslet.2009.10.043. Epub 2009 Oct 21. FEBS Lett. 2010. PMID: 19852959 Review.
Cited by
-
Lung Epithelial Cell Line Immune Responses to Pneumocystis.J Fungi (Basel). 2023 Jul 6;9(7):729. doi: 10.3390/jof9070729. J Fungi (Basel). 2023. PMID: 37504718 Free PMC article. Review.
-
Breakthrough Pneumocystis jirovecii Pneumonia in an Allogeneic Hematopoietic Stem Cell Transplant Recipient.Cureus. 2024 Jun 7;16(6):e61890. doi: 10.7759/cureus.61890. eCollection 2024 Jun. Cureus. 2024. PMID: 38978902 Free PMC article.
-
Targeting β-glucans, vital components of the Pneumocystis cell wall.Front Immunol. 2023 Feb 9;14:1094464. doi: 10.3389/fimmu.2023.1094464. eCollection 2023. Front Immunol. 2023. PMID: 36845149 Free PMC article. Review.
-
Pneumocystis.Cold Spring Harb Perspect Med. 2014 Nov 3;4(12):a019828. doi: 10.1101/cshperspect.a019828. Cold Spring Harb Perspect Med. 2014. PMID: 25367973 Free PMC article. Review.
-
Roles of the Mevalonate Pathway and Cholesterol Trafficking in Pulmonary Host Defense.Curr Mol Pharmacol. 2017;10(1):27-45. doi: 10.2174/1874467209666160112123603. Curr Mol Pharmacol. 2017. PMID: 26758950 Free PMC article.
References
-
- Beck JM, Rosen MJ, Peavy HH. Pulmonary complications of HIV infection. Report of the Fourth NHLBI Workshop. Am J Respir Crit Care Med. 2001;164:2120–6. - PubMed
-
- Thomas CF, Jr, Limper AH. Pneumocystis pneumonia: clinical presentation and diagnosis in patients with and without acquired immune deficiency syndrome. Semin Respir Infect. 1998;13:289–95. - PubMed
-
- Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–98. - PubMed
-
- Festic E, Gajic O, Limper AH, Aksamit TR. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest. 2005;128:573–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

