Loss of effector function of human cytolytic T lymphocytes is accompanied by major alterations in N- and O-glycosylation
- PMID: 22334653
- PMCID: PMC3322850
- DOI: 10.1074/jbc.M111.320820
Loss of effector function of human cytolytic T lymphocytes is accompanied by major alterations in N- and O-glycosylation
Abstract
Most human tumors are not eliminated by the immune system, and therapeutic vaccination shows poor results, a fact that can be explained at least partially by an immunosuppressive tumor microenvironment that is abundant in galectin-3. On cytolytic T lymphocyte (CTL) clones, maintained in culture by regular stimulation, recently activated CTLs present low effector functions. However, these functions are restored after a short treatment with LacNAc. The latter, which is in agreement with the glycoprotein-galectin lattice concept involving reduced motility, poses the question why galectin-3 ligands improve effector functions. We employed ultrasensitive MALDI-TOF-MS on resting and recently activated CTL clones combined with various glycosidase digestions and GC-MS linkage analyses. Our results showed that compared with the resting CTLs, the N-glycans of the recently activated CTLs consisted of (i) larger LacNAc oligomers of which a significant portion was longer than four-units and (ii) more multi-antennary structures. Interestingly, our results showed that the poly-LacNAc appeared to be equally distributed on all available N-glycan branches and not selectively enriched on a specific branch. The above structural alterations in the recently activated CTLs are expected to increase the galectin-3-LacNAc lattices and multivalent interactions and, therefore, reduce the motility of surface glycoproteins, such as the T-cell receptor. These findings suggest that the loss of effector functions on CTLs may be linked to reduced motility of surface glycoproteins. In addition, our results showed that recently activated CTLs had a reduced abundance of NeuAcα2,6-linked N-glycans and an increased abundance of disialylated core 1 and monosialylated core 2 O-glycan structures.
Figures


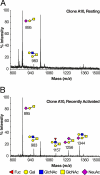
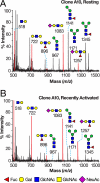
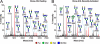
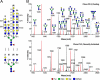

References
-
- Boon T., Coulie P. G., Van den Eynde B. J., van der Bruggen P. (2006) Human T cell responses against melanoma. Annu. Rev. Immunol. 24, 175–208 - PubMed
-
- Zippelius A., Batard P., Rubio-Godoy V., Bioley G., Liénard D., Lejeune F., Rimoldi D., Guillaume P., Meidenbauer N., Mackensen A., Rufer N., Lubenow N., Speiser D., Cerottini J. C., Romero P., Pittet M. J. (2004) Effector function of human tumor-specific CD8 T cells in melanoma lesions. A state of local functional tolerance. Cancer Res. 64, 2865–2873 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

