Synaptic released zinc promotes tau hyperphosphorylation by inhibition of protein phosphatase 2A (PP2A)
- PMID: 22334661
- PMCID: PMC3322889
- DOI: 10.1074/jbc.M111.309070
Synaptic released zinc promotes tau hyperphosphorylation by inhibition of protein phosphatase 2A (PP2A)
Abstract
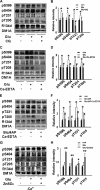
Hyperphosphorylated tau is the major component of neurofibrillary tangles in Alzheimer disease (AD), and the tangle distribution largely overlaps with zinc-containing glutamatergic neurons, suggesting that zinc released in synaptic terminals may play a role in tau phosphorylation. To explore this possibility, we treated cultured hippocampal slices or primary neurons with glutamate or Bic/4-AP to increase the synaptic activity with or without pretreatment of zinc chelators, and then detected the phosphorylation levels of tau. We found that glutamate or Bic/4-AP treatment caused tau hyperphosphorylation at multiple AD-related sites, including Ser-396, Ser-404, Thr-231, and Thr-205, while application of intracellular or extracellular zinc chelators, or blockade of zinc release by extracellular calcium omission almost abolished the synaptic activity-associated tau hyperphosphorylation. The zinc release and translocation of excitatory synapses in the hippocampus were detected, and zinc-induced tau hyperphosphorylation was also observed in cultured brain slices incubated with exogenously supplemented zinc. Tau hyperphosphorylation induced by synaptic activity was strongly associated with inactivation of protein phosphatase 2A (PP2A), and this inactivation can be reversed by pretreatment of zinc chelator. Together, these results suggest that synaptically released zinc promotes tau hyperphosphorylation through PP2A inhibition.
Figures


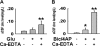



Similar articles
-
Zinc induces protein phosphatase 2A inactivation and tau hyperphosphorylation through Src dependent PP2A (tyrosine 307) phosphorylation.Neurobiol Aging. 2013 Mar;34(3):745-56. doi: 10.1016/j.neurobiolaging.2012.07.003. Epub 2012 Aug 11. Neurobiol Aging. 2013. PMID: 22892311
-
Effects of Porphyromonas gingivalis and Its Underlying Mechanisms on Alzheimer-Like Tau Hyperphosphorylation in Sprague-Dawley Rats.J Mol Neurosci. 2021 Jan;71(1):89-100. doi: 10.1007/s12031-020-01629-1. Epub 2020 Jun 16. J Mol Neurosci. 2021. PMID: 32557144
-
Protein phosphatase 2A restrains DLK signaling to promote proper Drosophila synaptic development and mammalian cortical neuron survival.Neurobiol Dis. 2022 Feb;163:105586. doi: 10.1016/j.nbd.2021.105586. Epub 2021 Dec 16. Neurobiol Dis. 2022. PMID: 34923110 Free PMC article.
-
Role of microtubule-associated protein tau phosphorylation in Alzheimer's disease.J Huazhong Univ Sci Technolog Med Sci. 2017 Jun;37(3):307-312. doi: 10.1007/s11596-017-1732-x. Epub 2017 Jun 6. J Huazhong Univ Sci Technolog Med Sci. 2017. PMID: 28585125 Review.
-
Tau pathology in Alzheimer disease and other tauopathies.Biochim Biophys Acta. 2005 Jan 3;1739(2-3):198-210. doi: 10.1016/j.bbadis.2004.09.008. Biochim Biophys Acta. 2005. PMID: 15615638 Review.
Cited by
-
HERV-W Envelope Triggers Abnormal Dopaminergic Neuron Process through DRD2/PP2A/AKT1/GSK3 for Schizophrenia Risk.Viruses. 2022 Jan 14;14(1):145. doi: 10.3390/v14010145. Viruses. 2022. PMID: 35062349 Free PMC article.
-
Behind the curtain of tauopathy: a show of multiple players orchestrating tau toxicity.Cell Mol Life Sci. 2016 Jan;73(1):1-21. doi: 10.1007/s00018-015-2042-8. Epub 2015 Sep 24. Cell Mol Life Sci. 2016. PMID: 26403791 Free PMC article. Review.
-
Zinc and the modulation of redox homeostasis.Free Radic Biol Med. 2012 Nov 1;53(9):1748-59. doi: 10.1016/j.freeradbiomed.2012.08.568. Epub 2012 Aug 25. Free Radic Biol Med. 2012. PMID: 22960578 Free PMC article. Review.
-
Zn2+ Aggravates Tau Aggregation and Neurotoxicity.Int J Mol Sci. 2019 Jan 23;20(3):487. doi: 10.3390/ijms20030487. Int J Mol Sci. 2019. PMID: 30678122 Free PMC article.
-
Metal Ion Effects on Aβ and Tau Aggregation.Int J Mol Sci. 2018 Jan 2;19(1):128. doi: 10.3390/ijms19010128. Int J Mol Sci. 2018. PMID: 29301328 Free PMC article. Review.
References
-
- Mount C., Downton C. (2006) Alzheimer disease: progress or profit? Nat. Med. 12, 780–784 - PubMed
-
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 261, 6084–6089 - PubMed
-
- Wang J. Z., Liu F. (2008) Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog. Neurobiol. 85, 148–175 - PubMed
-
- Bancher C., Brunner C., Lassmann H., Budka H., Jellinger K., Wiche G., Seitelberger F., Grundke-Iqbal I., Iqbal K., Wisniewski H. M. (1989) Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 477, 90–99 - PubMed
-
- Köpke E., Tung Y. C., Shaikh S., Alonso A. C., Iqbal K., Grundke-Iqbal I. (1993) Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J. Biol. Chem. 268, 24374–24384 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

