Extracellular low pH modulates phosphatidylserine-dependent phagocytosis in macrophages by increasing stabilin-1 expression
- PMID: 22334667
- PMCID: PMC3322863
- DOI: 10.1074/jbc.M111.310953
Extracellular low pH modulates phosphatidylserine-dependent phagocytosis in macrophages by increasing stabilin-1 expression
Abstract
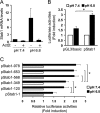
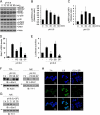
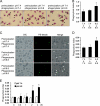
Microenvironmental acidosis is a common feature of inflammatory loci, in which clearance of apoptotic cells is necessary for the resolution of inflammation. Although it is known that a low pH environment affects immune function, its effect on apoptotic cell clearance by macrophages has not been fully investigated. Here, we show that treatment of macrophages with low pH medium resulted in increased expression of stabilin-1 out of several receptors, which are known to be involved in PS-dependent removal of apoptotic cells. Reporter assays showed that the -120/-1 region of the mouse stabilin-1 promoter was a low pH-responsive region and provided evidence that extracellular low pH mediated transcriptional activation of stabilin-1 via Ets-2. Furthermore, extracellular low pH activated JNK, thereby inducing translocation of Ets-2 into the nucleus. When macrophages were preincubated with low pH medium, phagocytosis of phosphatidylserine-exposed red blood cells and phosphatidylserine-coated beads by macrophages was enhanced. Blockade of stabilin-1 in macrophages abolished the enhancement of phagocytic activity by low pH. Thus, our results demonstrate that a low pH microenvironment up-regulates stabilin-1 expression in macrophages, thereby modulating the phagocytic capacity of macrophages, and suggest roles for stabilin-1 and Ets-2 in the maintenance of tissue homeostasis by the immune system.
Figures

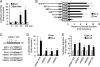

References
-
- Dubos R. J. (1955) The micro-environment of inflammation or Metchnikoff revisited. Lancet 269, 1–5 - PubMed
-
- Grimshaw M. J., Balkwill F. R. (2001) Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation. A potential mechanism. Eur. J. Immunol. 31, 480–489 - PubMed
-
- Edlow D. W., Sheldon W. H. (1971) The pH of inflammatory exudates. Proc. Soc. Exp. Biol. Med. 137, 1328–1332 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

