LytA, major autolysin of Streptococcus pneumoniae, requires access to nascent peptidoglycan
- PMID: 22334685
- PMCID: PMC3322828
- DOI: 10.1074/jbc.M111.318584
LytA, major autolysin of Streptococcus pneumoniae, requires access to nascent peptidoglycan
Abstract
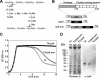
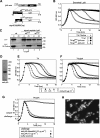
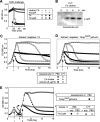
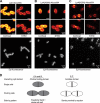
The pneumococcal autolysin LytA is a virulence factor involved in autolysis as well as in fratricidal- and penicillin-induced lysis. In this study, we used biochemical and molecular biological approaches to elucidate which factors control the cytoplasmic translocation and lytic activation of LytA. We show that LytA is mainly localized intracellularly, as only a small fraction was found attached to the extracellular cell wall. By manipulating the extracellular concentration of LytA, we found that the cells were protected from lysis during exponential growth, but not in the stationary phase, and that a defined threshold concentration of extracellular LytA dictates the onset of autolysis. Stalling growth through nutrient depletion, or the specific arrest of cell wall synthesis, sensitized cells for LytA-mediated lysis. Inhibition of cell wall association via the choline binding domain of an exogenously added enzymatically inactive form of LytA revealed a potential substrate for the amidase domain within the cell wall where the formation of nascent peptidoglycan occurs.
Figures



References
-
- Neufeld F. (1900) Über eine specifische bakteriolytische Wirkung der Galle. Z. Hyg. Infektionskr. 34, 454–464
-
- Tomasz A., Albino A., Zanati E. (1970) Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature 227, 138–140 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

