The transmembrane prolines of the mitochondrial ADP/ATP carrier are involved in nucleotide binding and transport and its biogenesis
- PMID: 22334686
- PMCID: PMC3322973
- DOI: 10.1074/jbc.M111.320697
The transmembrane prolines of the mitochondrial ADP/ATP carrier are involved in nucleotide binding and transport and its biogenesis
Abstract
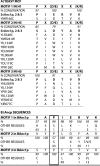
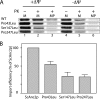
The mitochondrial ADP/ATP carrier (Ancp) is a paradigm of the mitochondrial carrier family, which allows cross-talk between mitochondria, where cell energy is mainly produced, and cytosol, where cell energy is mainly consumed. The members of this family share numerous structural and functional characteristics. Resolution of the atomic structure of the bovine Ancp, in a complex with one of its specific inhibitors, revealed interesting features and suggested the involvement of some particular residues in the movements of the protein to perform translocation of nucleotides from one side of the membrane to the other. They correspond to three prolines located in the odd-numbered transmembrane helices (TMH), Pro-27, Pro-132, and Pro-229. The corresponding residues of the yeast Ancp (Pro-43, Ser-147, and Pro-247) were mutated into alanine or leucine, one at a time and analysis of the various mutants evidenced a crucial role of Pro-43 and Pro-247 during nucleotide transport. Beside, replacement of Ser-147 with proline does not inactivate Ancp and this is discussed in view of the conservation of the three prolines at equivalent positions in the Ancp sequences. These prolines belong to the signature sequences of the mitochondrial carriers and we propose they play a dual role in the mitochondrial ADP/ATP carrier function and biogenesis. Unexpectedly their mutations cause more general effects on mitochondrial biogenesis and morphology, as evidenced by measurements of respiratory rates, cytochrome contents, and also clearly highlighted by fluorescence microscopy.
Figures




References
-
- Pebay-Peyroula E., Dahout-Gonzalez C., Kahn R., Trézéguet V., Lauquin G. J.-M., Brandolin G. (2003) Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426, 39–44 - PubMed
-
- Morozzo Della Rocca B., Miniero D. V., Tasco G., Dolce V., Falconi M., Ludovico A., Cappello A. R., Sanchez P., Stipani I., Casadio R., Desideri A., Palmieri F. (2005) Substrate-induced conformational changes of the mitochondrial oxoglutarate carrier. A spectroscopic and molecular modelling study. Mol. Membr. Biol. 22, 443–452 - PubMed
-
- Tonazzi A., Giangregorio N., Palmieri F., Indiveri C. (2005) Relationships of cysteine and lysine residues with the substrate binding site of the mitochondrial ornithine/citrulline carrier. An inhibition kinetic approach combined with the analysis of the homology structural model. Biochim. Biophys. Acta 1718, 53–60 - PubMed
-
- Kunji E. R., Robinson A. J. (2006) The conserved substrate binding site of mitochondrial carriers. Biochim. Biophys. Acta 1757, 1237–1248 - PubMed
-
- Perchiniak E., Lawrence S. A., Kasten S., Woodard B. A., Taylor S. M., Moran R. G. (2007) Probing the mechanism of the hamster mitochondrial folate transporter by mutagenesis and homology modeling. Biochemistry 46, 1557–1567 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

