Rapid aquaporin translocation regulates cellular water flow: mechanism of hypotonicity-induced subcellular localization of aquaporin 1 water channel
- PMID: 22334691
- PMCID: PMC3322852
- DOI: 10.1074/jbc.M111.329219
Rapid aquaporin translocation regulates cellular water flow: mechanism of hypotonicity-induced subcellular localization of aquaporin 1 water channel
Abstract
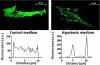
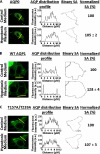
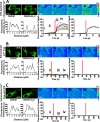
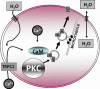
The control of cellular water flow is mediated by the aquaporin (AQP) family of membrane proteins. The structural features of the family and the mechanism of selective water passage through the AQP pore are established, but there remains a gap in our knowledge of how water transport is regulated. Two broad possibilities exist. One is controlling the passage of water through the AQP pore, but this only has been observed as a phenomenon in some plant and microbial AQPs. An alternative is controlling the number of AQPs in the cell membrane. Here, we describe a novel pathway in mammalian cells whereby a hypotonic stimulus directly induces intracellular calcium elevations through transient receptor potential channels, which trigger AQP1 translocation. This translocation, which has a direct role in cell volume regulation, occurs within 30 s and is dependent on calmodulin activation and phosphorylation of AQP1 at two threonine residues by protein kinase C. This direct mechanism provides a rationale for the changes in water transport that are required in response to constantly changing local cellular water availability. Moreover, because calcium is a pluripotent and ubiquitous second messenger in biological systems, the discovery of its role in the regulation of AQP translocation has ramifications for diverse physiological and pathophysiological processes, as well as providing an explanation for the rapid regulation of water flow that is necessary for cell homeostasis.
Figures

Similar articles
-
The speed of swelling kinetics modulates cell volume regulation and calcium signaling in astrocytes: A different point of view on the role of aquaporins.Glia. 2016 Jan;64(1):139-54. doi: 10.1002/glia.22921. Epub 2015 Sep 28. Glia. 2016. PMID: 26413835 Free PMC article.
-
An emerging consensus on aquaporin translocation as a regulatory mechanism.Mol Membr Biol. 2013 Feb;30(1):1-12. doi: 10.3109/09687688.2012.743194. Epub 2012 Nov 21. Mol Membr Biol. 2013. PMID: 23170905 Review.
-
Aquaporin 1 mediates early responses to osmotic stimuli in endothelial cells via the calmodulin pathway.FEBS Open Bio. 2021 Jan;11(1):75-84. doi: 10.1002/2211-5463.13020. Epub 2020 Nov 24. FEBS Open Bio. 2021. PMID: 33125833 Free PMC article.
-
Identification and Molecular Mechanisms of the Rapid Tonicity-induced Relocalization of the Aquaporin 4 Channel.J Biol Chem. 2015 Jul 3;290(27):16873-81. doi: 10.1074/jbc.M115.646034. Epub 2015 May 26. J Biol Chem. 2015. PMID: 26013827 Free PMC article.
-
Functional domains of aquaporin-1: keys to physiology, and targets for drug discovery.Curr Pharm Des. 2007;13(31):3212-21. doi: 10.2174/138161207782341349. Curr Pharm Des. 2007. PMID: 18045170 Review.
Cited by
-
Ions, the Movement of Water and the Apoptotic Volume Decrease.Front Cell Dev Biol. 2020 Nov 25;8:611211. doi: 10.3389/fcell.2020.611211. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33324655 Free PMC article. Review.
-
Signaling Mechanisms and Pharmacological Modulators Governing Diverse Aquaporin Functions in Human Health and Disease.Int J Mol Sci. 2022 Jan 26;23(3):1388. doi: 10.3390/ijms23031388. Int J Mol Sci. 2022. PMID: 35163313 Free PMC article. Review.
-
Aquaporin-1 translocation and degradation mediates the water transportation mechanism of acetazolamide.PLoS One. 2012;7(9):e45976. doi: 10.1371/journal.pone.0045976. Epub 2012 Sep 21. PLoS One. 2012. PMID: 23029347 Free PMC article.
-
The Emerging Roles of Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 in Skeletal Muscle Redox Signaling and Metabolism.Antioxid Redox Signal. 2019 Dec 20;31(18):1371-1410. doi: 10.1089/ars.2018.7678. Epub 2019 Nov 1. Antioxid Redox Signal. 2019. PMID: 31588777 Free PMC article. Review.
-
Expression of aquaporin1, a water channel protein, in cytoplasm is negatively correlated with prognosis of breast cancer patients.Oncotarget. 2016 Feb 16;7(7):8143-54. doi: 10.18632/oncotarget.6994. Oncotarget. 2016. PMID: 26812884 Free PMC article.
References
-
- Benga G., Popescu O., Borza V., Pop V. I., Muresan A., Mocsy I., Brain A., Wrigglesworth J. M. (1986) Water permeability in human erythrocytes: Identification of membrane proteins involved in water transport. Eur. J. Cell Biol. 41, 252–262 - PubMed
-
- de Groot B. L., Frigato T., Helms V., Grubmüller H. (2003) The mechanism of proton exclusion in the aquaporin-1 water channel. J. Mol. Biol. 333, 279–293 - PubMed
-
- de Groot B. L., Grubmüller H. (2001) Water permeation across biological membranes: Mechanism and dynamics of aquaporin-1 and GlpF. Science 294, 2353–2357 - PubMed
-
- Walz T., Fujiyoshi Y., Engel A. (2009) The AQP structure and functional implications. Handb. Exp. Pharmacol. 190, 31–56 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

