Rapid aquaporin translocation regulates cellular water flow: mechanism of hypotonicity-induced subcellular localization of aquaporin 1 water channel
- PMID: 22334691
- PMCID: PMC3322852
- DOI: 10.1074/jbc.M111.329219
Rapid aquaporin translocation regulates cellular water flow: mechanism of hypotonicity-induced subcellular localization of aquaporin 1 water channel
Abstract
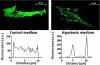
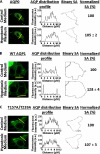
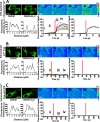
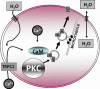
The control of cellular water flow is mediated by the aquaporin (AQP) family of membrane proteins. The structural features of the family and the mechanism of selective water passage through the AQP pore are established, but there remains a gap in our knowledge of how water transport is regulated. Two broad possibilities exist. One is controlling the passage of water through the AQP pore, but this only has been observed as a phenomenon in some plant and microbial AQPs. An alternative is controlling the number of AQPs in the cell membrane. Here, we describe a novel pathway in mammalian cells whereby a hypotonic stimulus directly induces intracellular calcium elevations through transient receptor potential channels, which trigger AQP1 translocation. This translocation, which has a direct role in cell volume regulation, occurs within 30 s and is dependent on calmodulin activation and phosphorylation of AQP1 at two threonine residues by protein kinase C. This direct mechanism provides a rationale for the changes in water transport that are required in response to constantly changing local cellular water availability. Moreover, because calcium is a pluripotent and ubiquitous second messenger in biological systems, the discovery of its role in the regulation of AQP translocation has ramifications for diverse physiological and pathophysiological processes, as well as providing an explanation for the rapid regulation of water flow that is necessary for cell homeostasis.
Figures

References
-
- Benga G., Popescu O., Borza V., Pop V. I., Muresan A., Mocsy I., Brain A., Wrigglesworth J. M. (1986) Water permeability in human erythrocytes: Identification of membrane proteins involved in water transport. Eur. J. Cell Biol. 41, 252–262 - PubMed
-
- de Groot B. L., Frigato T., Helms V., Grubmüller H. (2003) The mechanism of proton exclusion in the aquaporin-1 water channel. J. Mol. Biol. 333, 279–293 - PubMed
-
- de Groot B. L., Grubmüller H. (2001) Water permeation across biological membranes: Mechanism and dynamics of aquaporin-1 and GlpF. Science 294, 2353–2357 - PubMed
-
- Walz T., Fujiyoshi Y., Engel A. (2009) The AQP structure and functional implications. Handb. Exp. Pharmacol. 190, 31–56 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

