Neisseria gonorrhoeae virulence factor NG1686 is a bifunctional M23B family metallopeptidase that influences resistance to hydrogen peroxide and colony morphology
- PMID: 22334697
- PMCID: PMC3322868
- DOI: 10.1074/jbc.M111.338830
Neisseria gonorrhoeae virulence factor NG1686 is a bifunctional M23B family metallopeptidase that influences resistance to hydrogen peroxide and colony morphology
Abstract
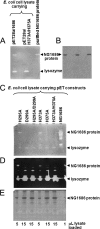
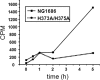
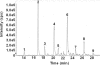
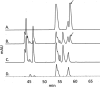
Symptomatic gonococcal infection, caused exclusively by the human-specific pathogen Neisseria gonorrhoeae (the gonococcus), is characterized by the influx of polymorphonuclear leukocytes (PMNs) to the site of infection. Although PMNs possess a potent antimicrobial arsenal comprising both oxidative and non-oxidative killing mechanisms, gonococci survive this interaction, suggesting that the gonococcus has evolved many defenses against PMN killing. We previously identified the NG1686 protein as a gonococcal virulence factor that protects against both non-oxidative PMN-mediated killing and oxidative killing by hydrogen peroxide. In this work, we show that deletion of ng1686 affects gonococcal colony morphology but not cell morphology and that overexpression of ng1686 does not confer enhanced survival to hydrogen peroxide on gonococci. NG1686 contains M23B endopeptidase active sites found in proteins that cleave bacterial cell wall peptidoglycan. Strains of N. gonorrhoeae expressing mutant NG1686 proteins with substitutions in many, but not all, conserved metallopeptidase active sites recapitulated the hydrogen peroxide sensitivity and altered colony morphology of the Δng1686 mutant strain. We showed that purified NG1686 protein degrades peptidoglycan in vitro and that mutations in many conserved active site residues abolished its degradative activity. Finally, we demonstrated that NG1686 possesses both dd-carboxypeptidase and endopeptidase activities. We conclude that the NG1686 protein is a M23B peptidase with dual activities that targets the cell wall to affect colony morphology and resistance to hydrogen peroxide and PMN-mediated killing.
Figures





References
-
- World Health Organization (2011) Emergence of multi-drug resistant Neisseria gonorrhoeae. Threat of global rise in untreatable sexually transmitted disease. in WHO/RHR/11.4 Department of Reproductive Health and Research, World Health Organization, Geneva
-
- Hook E. W., 3rd, Holmes K. K. (1985) Gonococcal infections. Ann. Int. Med. 102, 229–243 - PubMed
-
- Dokter W. H., Dijkstra A. J., Koopmans S. B., Stulp B. K., Keck W., Halie M. R., Vellenga E. (1994) G(Anh)MTetra, a natural bacterial cell wall breakdown product, induces interleukin-1 β and interleukin-6 expression in human monocytes. A study of the molecular mechanisms involved in inflammatory cytokine expression. J. Biol. Chem. 269, 4201–4206 - PubMed
-
- Melly M. A., McGee Z. A., Rosenthal R. S. (1984) Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian-tube mucosa. J. Infect. Dis. 149, 378–386 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

