First and second generation γ-secretase modulators (GSMs) modulate amyloid-β (Aβ) peptide production through different mechanisms
- PMID: 22334705
- PMCID: PMC3320929
- DOI: 10.1074/jbc.M111.305227
First and second generation γ-secretase modulators (GSMs) modulate amyloid-β (Aβ) peptide production through different mechanisms
Abstract
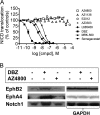
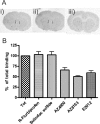
γ-Secretase-mediated cleavage of amyloid precursor protein (APP) results in the production of Alzheimer disease-related amyloid-β (Aβ) peptides. The Aβ42 peptide in particular plays a pivotal role in Alzheimer disease pathogenesis and represents a major drug target. Several γ-secretase modulators (GSMs), such as the nonsteroidal anti-inflammatory drugs (R)-flurbiprofen and sulindac sulfide, have been suggested to modulate the Alzheimer-related Aβ production by targeting the APP. Here, we describe novel GSMs that are selective for Aβ modulation and do not impair processing of Notch, EphB2, or EphA4. The GSMs modulate Aβ both in cell and cell-free systems as well as lower amyloidogenic Aβ42 levels in the mouse brain. Both radioligand binding and cellular cross-competition experiments reveal a competitive relationship between the AstraZeneca (AZ) GSMs and the established second generation GSM, E2012, but a noncompetitive interaction between AZ GSMs and the first generation GSMs (R)-flurbiprofen and sulindac sulfide. The binding of a (3)H-labeled AZ GSM analog does not co-localize with APP but overlaps anatomically with a γ-secretase targeting inhibitor in rodent brains. Combined, these data provide compelling evidence of a growing class of in vivo active GSMs, which are selective for Aβ modulation and have a different mechanism of action compared with the original class of GSMs described.
Figures





Similar articles
-
Second generation γ-secretase modulators exhibit different modulation of Notch β and Aβ production.J Biol Chem. 2012 Sep 21;287(39):32640-50. doi: 10.1074/jbc.M112.376541. Epub 2012 Jul 31. J Biol Chem. 2012. PMID: 22851182 Free PMC article.
-
Potent γ-secretase inhibitors/modulators interact with amyloid-β fibrils but do not inhibit fibrillation: a high-resolution NMR study.Biochem Biophys Res Commun. 2014 May 16;447(4):590-5. doi: 10.1016/j.bbrc.2014.04.041. Epub 2014 Apr 18. Biochem Biophys Res Commun. 2014. PMID: 24747079
-
γ-Secretase modulator in Alzheimer's disease: shifting the end.J Alzheimers Dis. 2012;31(4):685-96. doi: 10.3233/JAD-2012-120751. J Alzheimers Dis. 2012. PMID: 22710916 Review.
-
Amyloid beta 42 peptide (Abeta42)-lowering compounds directly bind to Abeta and interfere with amyloid precursor protein (APP) transmembrane dimerization.Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14597-602. doi: 10.1073/pnas.1003026107. Epub 2010 Aug 2. Proc Natl Acad Sci U S A. 2010. PMID: 20679249 Free PMC article.
-
Development and mechanism of γ-secretase modulators for Alzheimer's disease.Biochemistry. 2013 May 14;52(19):3197-216. doi: 10.1021/bi400377p. Epub 2013 May 2. Biochemistry. 2013. PMID: 23614767 Free PMC article. Review.
Cited by
-
Design and synthesis of novel methoxypyridine-derived gamma-secretase modulators.Bioorg Med Chem. 2020 Nov 15;28(22):115734. doi: 10.1016/j.bmc.2020.115734. Epub 2020 Sep 1. Bioorg Med Chem. 2020. PMID: 33007551 Free PMC article.
-
Initial Optimization of a New Series of γ-Secretase Modulators Derived from a Triterpene Glycoside.ACS Med Chem Lett. 2012 Aug 29;3(11):908-13. doi: 10.1021/ml300256p. eCollection 2012 Nov 8. ACS Med Chem Lett. 2012. PMID: 24900406 Free PMC article.
-
Aftins increase amyloid-β42, lower amyloid-β38, and do not alter amyloid-β40 extracellular production in vitro: toward a chemical model of Alzheimer's disease?J Alzheimers Dis. 2013;35(1):107-20. doi: 10.3233/JAD-121777. J Alzheimers Dis. 2013. PMID: 23364140 Free PMC article.
-
γ-Secretase in Alzheimer's disease.Exp Mol Med. 2022 Apr;54(4):433-446. doi: 10.1038/s12276-022-00754-8. Epub 2022 Apr 8. Exp Mol Med. 2022. PMID: 35396575 Free PMC article. Review.
-
Critical analysis of the use of β-site amyloid precursor protein-cleaving enzyme 1 inhibitors in the treatment of Alzheimer's disease.Degener Neurol Neuromuscul Dis. 2014 Jan 22;4:1-19. doi: 10.2147/DNND.S41056. eCollection 2014. Degener Neurol Neuromuscul Dis. 2014. PMID: 32669897 Free PMC article. Review.
References
-
- Walsh D. M., Selkoe D. J. (2004) Deciphering the molecular basis of memory failure in Alzheimer disease. Neuron 44, 181–193 - PubMed
-
- Lundkvist J., Näslund J. (2007) γ-Secretase. A complex target for Alzheimer disease. Curr. Opin. Pharmacol. 7, 112–118 - PubMed
-
- Weggen S., Eriksen J. L., Das P., Sagi S. A., Wang R., Pietrzik C. U., Findlay K. A., Smith T. E., Murphy M. P., Bulter T., Kang D. E., Marquez-Sterling N., Golde T. E., Koo E. H. (2001) A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

