Efficacy and safety of a fixed-dose combination of mometasone furoate and formoterol fumarate in subjects with moderate to very severe COPD: results from a 52-week Phase III trial
- PMID: 22334768
- PMCID: PMC3276256
- DOI: 10.2147/COPD.S27319
Efficacy and safety of a fixed-dose combination of mometasone furoate and formoterol fumarate in subjects with moderate to very severe COPD: results from a 52-week Phase III trial
Erratum in
- Int J Chron Obstruct Pulmon Dis. 2012;7:765
Abstract
Background: A clinical trial of mometasone furoate/formoterol fumarate (MF/F) administered via a metered-dose inhaler in subjects with moderate to very severe chronic obstructive pulmonary disease (COPD) investigated the efficacy and safety of a fixed-dose combination of MF/F.
Methods: This multicenter, double-blind, placebo-controlled trial had a 26-week treatment period and a 26-week safety extension. Subjects (n = 1055; ≥40 years) were current or ex- smokers randomized to twice-daily treatment with inhaled MF/F 400/10 μg, MF/F 200/10 μg, MF 400 μg, F 10 μg, or placebo. The coprimary endpoints of the trial were mean changes from baseline in forced expiratory volume in 1 second (FEV(1)) over 0-12 hours (AUC(0-12) FEV(1)) with MF/F versus MF, and in morning predose FEV(1) with MF/F versus F. Key secondary endpoints were quality of life (Saint George's Respiratory Questionnaire [SGRQ]), symptom-free nights, and partly stable COPD at 26 weeks, as well as time to first COPD exacerbation.
Results: Significant improvements in FEV(1) AUC(0-12) occurred at endpoint with MF/F 400/10 and MF/F 200/10 versus MF 400 (P ≤ 0.007). Significant bronchodilation occurred in 5 minutes with MF/F, and serial spirometry demonstrated sustained FEV(1) improvements with MF/F over the treatment period. Significant improvements in morning predose FEV(1) occurred with both MF/F doses, and these effects were further investigated by excluding results for subjects whose morning FEV(1) data were collected >2 days after the last dose of study treatment. Improvements in SGRQ total scores surpassed the minimum clinically important difference of at least 4 units with MF/F 400/10. MF/F 400/10 significantly reduced the time-to-first COPD exacerbation. Similar proportions of subjects in all five treatment groups reported treatment-emergent adverse events. Rates of pneumonia were low (≤1.0%) across treatment groups.
Conclusion: MF/F 400/10 μg twice daily was shown to be an effective therapy for patients with moderate to very severe COPD, and both MF/F 400/10 μg twice daily and MF/F 200/10 μg twice daily were well tolerated.
Trial registration: ClinicalTrials.gov NCT00383435.
Keywords: FEV1; bronchodilator; chronic obstructive pulmonary disease; exacerbation; inhaled corticosteroid; spirometry.
Figures
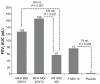
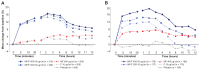

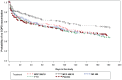
References
-
- American Thoracic Society/European Respiratory Society Task Force. Standards for the Diagnosis and Management of Patients with COPD. [Accessed July 26, 2011]. Available at: http://www.thoracic.org/clinical/copd-guidelines/resources/copddoc.pdf.
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) [Accessed July 21, 2011]. Available at: http://www.goldcopd.org/
-
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. - PubMed
-
- Doherty DE. A review of the role of FEV1 in the COPD paradigm. COPD. 2008;5:310–318. - PubMed

