Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed-dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52-week placebo-controlled trials
- PMID: 22334770
- PMCID: PMC3276258
- DOI: 10.2147/COPD.S29444
Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed-dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52-week placebo-controlled trials
Abstract
Background: The clinical efficacy and safety of a mometasone furoate/formoterol fumarate (MF/F) fixed-dose combination formulation administered via a metered-dose inhaler was investigated in patients with moderate to very severe chronic obstructive pulmonary disease (COPD).
Methods: Two 52-week, multicenter, double-blind, placebo-controlled trials with identical study designs were conducted in current or ex-smokers (aged ≥40 years), and pooled study results are presented herein. Subjects (n = 2251) were randomized to 26 weeks of twice-daily treatment with MF/F 400/10 μg, MF/F 200/10 μg, MF 400 μg, F 10 μg, or placebo. After the 26-week treatment period, placebo subjects completed the trial and 75% of subjects on active treatment entered a 26-week safety extension. Coprimary efficacy variables were mean changes in forced expiratory volume in one second (FEV(1)), area under the curve from 0 to 12 hours postdose (AUC(0-12 h)), and morning predose/trough FEV(1) from baseline to the week 13 endpoint. Key secondary efficacy variables were St George's Respiratory Questionnaire scores, symptom-free nights, time-to-first exacerbation, and partly stable COPD at the week 26 endpoint.
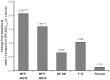
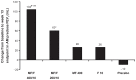
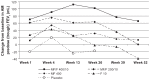
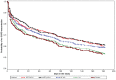
Results: In the 26-week treatment period, significantly greater increases in FEV(1) AUC(0-12 h) occurred with MF/F 400/10 versus MF 400 and placebo at the week 13 and week 26 endpoints (P ≤ 0.032). These increases were over three-fold greater with MF/F 400/10 than with MF 400. Also, significantly greater increases in morning predose/trough FEV(1) occurred with MF/F 400/10 versus F 10 and placebo at the week 13 endpoint (P < 0.05). The increase was four-fold greater with MF/F 400/10 than with F 10. All active treatment groups achieved minimum clinically important differences from baseline (>4 units) in St George's Respiratory Questionnaire scores at week 26. Symptom-free nights increased by ≥14% in the MF/F 400/10, MF 400, and F 10 groups (P ≤ 0.033 versus placebo). The incidence of exacerbations was lower in the MF/F groups (≤33.3%) than it was in the MF, formoterol, and placebo groups (≥33.8%) over the 26-week treatment period. The incidence of adverse events was similar in the active-treated and placebo-treated subjects across 26 weeks of treatment. Over the 1-year study period, there were no notable differences in the incidence or types of adverse events between the MF/F 400/10 and MF/F 200/10 groups compared with the MF or formoterol groups. Differences in rates of individual treatment-emergent adverse events were <3% between treatment groups. Rates of pneumonia were low (≤2%) across all treatment groups.
Conclusion: Patients treated with MF/F demonstrated significant improvements in lung function, health status, and exacerbation rates. Although significant improvements were seen with both doses, a trend showing a dose-response effect was observed in the lung function measurements.
Trial registration: ClinicalTrials.gov NCT00383435 NCT00383721.
Keywords: COPD; bronchodilator; exacerbation; inhaled corticosteroid; spirometry.
Figures


References
-
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American college of physicians, American college of chest physicians, American thoracic society, and European respiratory society. Ann Intern Med. 2011;155:179–191. - PubMed
-
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. - PubMed
-
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. - PubMed
-
- Aalbers R, Ayres J, Backer V, et al. Formoterol in patients with chronic obstructive pulmonary disease: a randomized, controlled, 3-month trial. Eur Respir J. 2002;19:936–943. - PubMed
-
- Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957–965. - PubMed

