Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer
- PMID: 22334787
- PMCID: PMC3278229
- DOI: 10.2147/IJN.S26538
Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer
Abstract
Background: Realizing the therapeutic benefits of quercetin is mostly hampered by its low water solubility and poor absorption. In light of the advantages of nanovehicles in the delivery of flavanoids, we aimed to deliver quercetin perorally with nanomicelles made from the diblock copolymer, polyethylene glycol (PEG)-derivatized phosphatidylethanolamine (PE).
Methods: Quercetin-loaded nanomicelles were prepared by using the film casting method, and were evaluated in terms of drug incorporation efficiency, micelle size, interaction with Caco-2 cells, and anticancer activity in the A549 lung cancer cell line and murine xenograft model.
Results: The incorporation efficiency into the nanomicelles was ≥88.9% when the content of quercetin was up to 4% w/w, with sizes of 15.4-18.5 nm and polydispersity indices of <0.250. Solubilization of quercetin by the nanomicelles increased its aqueous concentration by 110-fold. The quercetin nanomicelles were stable when tested in simulated gastric (pH 1.2) and intestinal (pH 7.4) fluids, and were non-toxic to the Caco-2 cells as reflected by reversible reduction in transepithelial electrical resistance and ≤25% lactose dehydrogenase release. The anticancer activity of quercetin could be significantly improved over the free drug through the nanomicellar formulation when tested using the A549 cancer cell line and murine xenograft model. The nanomicellar quercetin formulation was well tolerated by the tumor-bearing animals, with no significant weight loss observed at the end of the 10-week study period.
Conclusion: A stable PEG-PE nanomicellar formulation of quercetin was developed with enhanced peroral anticancer activity and no apparent toxicity to the intestinal epithelium.
Keywords: PEG-PE; lung cancer; peroral drug delivery; polymeric micelles; quercetin.
Figures
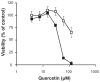
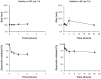


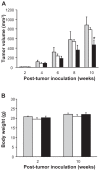
 ), quercetin in ethanol-based suspension (□) or nanomicelles (■). Notes: *represents P < 0.05 compared to control and quercetin oral suspension.
), quercetin in ethanol-based suspension (□) or nanomicelles (■). Notes: *represents P < 0.05 compared to control and quercetin oral suspension.Similar articles
-
Formulation of Nanomicelles to Improve the Solubility and the Oral Absorption of Silymarin.Molecules. 2019 Apr 30;24(9):1688. doi: 10.3390/molecules24091688. Molecules. 2019. PMID: 31052197 Free PMC article.
-
Enhancing the anti-colon cancer activity of quercetin by self-assembled micelles.Int J Nanomedicine. 2015 Mar 16;10:2051-63. doi: 10.2147/IJN.S75550. eCollection 2015. Int J Nanomedicine. 2015. PMID: 25844036 Free PMC article.
-
pH-Responsive Block Copolymer Micelles of Temsirolimus: Preparation, Characterization and Antitumor Activity Evaluation.Int J Nanomedicine. 2024 Sep 23;19:9821-9841. doi: 10.2147/IJN.S469913. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39345910 Free PMC article.
-
Nanomicellar carriers for targeted delivery of anticancer agents.Ther Deliv. 2014 Jan;5(1):53-68. doi: 10.4155/tde.13.135. Ther Deliv. 2014. PMID: 24341817 Free PMC article. Review.
-
Nano Drug Delivery Strategies for an Oral Bioenhanced Quercetin Formulation.Eur J Drug Metab Pharmacokinet. 2023 Sep;48(5):495-514. doi: 10.1007/s13318-023-00843-7. Epub 2023 Jul 31. Eur J Drug Metab Pharmacokinet. 2023. PMID: 37523008 Review.
Cited by
-
Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals.Chin J Nat Med. 2015 Sep;13(9):641-52. doi: 10.1016/S1875-5364(15)30061-3. Chin J Nat Med. 2015. PMID: 26412423 Free PMC article. Review.
-
Exploring the Remarkable Chemotherapeutic Potential of Polyphenolic Antioxidants in Battling Various Forms of Cancer.Molecules. 2023 Apr 14;28(8):3475. doi: 10.3390/molecules28083475. Molecules. 2023. PMID: 37110709 Free PMC article. Review.
-
Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health.ACS Omega. 2020 May 14;5(20):11849-11872. doi: 10.1021/acsomega.0c01818. eCollection 2020 May 26. ACS Omega. 2020. PMID: 32478277 Free PMC article.
-
Flavonoid Nanoparticles: A Promising Approach for Cancer Therapy.Biomolecules. 2020 Sep 2;10(9):1268. doi: 10.3390/biom10091268. Biomolecules. 2020. PMID: 32887473 Free PMC article. Review.
-
A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids.Molecules. 2023 Jun 25;28(13):4982. doi: 10.3390/molecules28134982. Molecules. 2023. PMID: 37446644 Free PMC article. Review.
References
-
- National Comprehensive Cancer Network. Clinical Practice Guidelines: Non-small cell lung cancer. 2008. [Accessed September 19, 2008]. Available from: http://www.nccn.org/professionals/physician_gls/PDF/nscl.pdf.
-
- Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–249. - PubMed
-
- Teicher BA, Andrews PA. Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials and Approval. 2nd ed. Totowa, NJ: Humana Press; 2004.
-
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. - PubMed
-
- Leonarduzzi G, Testa G, Sottero B, Gamba P, Poli G. Design and development of nanovehicle-based delivery systems for preventive or therapeutic supplementation with flavonoids. Curr Med Chem. 2010;17(1):74–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

