Key role for ubiquitin protein modification in TGFβ signal transduction
- PMID: 22335355
- PMCID: PMC3339547
- DOI: 10.3109/03009734.2012.654858
Key role for ubiquitin protein modification in TGFβ signal transduction
Abstract
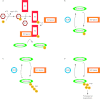
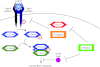
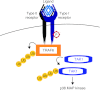
The transforming growth factor β (TGFβ) superfamily of signal transduction molecules plays crucial roles in the regulation of cell behavior. TGFβ regulates gene transcription through Smad proteins and signals via non-Smad pathways. The TGFβ pathway is strictly regulated, and perturbations lead to tumorigenesis. Several pathway components are known to be targeted for proteasomal degradation via ubiquitination by E3 ligases. Smurfs are well known negative regulators of TGFβ, which function as E3 ligases recruited by adaptors such as I-Smads. TGFβ signaling can also be enhanced by E3 ligases, such as Arkadia, that target repressors for degradation. It is becoming clear that E3 ligases often target multiple pathways, thereby acting as mediators of signaling cross-talk. Regulation via ubiquitination involves a complex network of E3 ligases, adaptor proteins, and deubiquitinating enzymes (DUBs), the last-mentioned acting by removing ubiquitin from its targets. Interestingly, also non-degradative ubiquitin modifications are known to play important roles in TGFβ signaling. Ubiquitin modifications thus play a key role in TGFβ signal transduction, and in this review we provide an overview of known players, focusing on recent advances.
Figures


Similar articles
-
E3 Ubiquitin Ligases: Key Regulators of TGFβ Signaling in Cancer Progression.Int J Mol Sci. 2021 Jan 6;22(2):476. doi: 10.3390/ijms22020476. Int J Mol Sci. 2021. PMID: 33418880 Free PMC article. Review.
-
Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases.J Biol Chem. 2005 Jun 10;280(23):22115-23. doi: 10.1074/jbc.M414027200. Epub 2005 Apr 6. J Biol Chem. 2005. PMID: 15817471
-
Regulation of the TGFbeta signalling pathway by ubiquitin-mediated degradation.Oncogene. 2004 Mar 15;23(11):2071-8. doi: 10.1038/sj.onc.1207412. Oncogene. 2004. PMID: 15021894 Review.
-
Regulation of TGF-beta family signaling by E3 ubiquitin ligases.Cancer Sci. 2008 Nov;99(11):2107-12. doi: 10.1111/j.1349-7006.2008.00925.x. Epub 2008 Sep 18. Cancer Sci. 2008. PMID: 18808420 Free PMC article. Review.
-
OTUB1 enhances TGFβ signalling by inhibiting the ubiquitylation and degradation of active SMAD2/3.Nat Commun. 2013;4:2519. doi: 10.1038/ncomms3519. Nat Commun. 2013. PMID: 24071738 Free PMC article.
Cited by
-
The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies.Front Mol Biosci. 2021 May 5;8:593310. doi: 10.3389/fmolb.2021.593310. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026818 Free PMC article. Review.
-
TRAF6 promotes TGFβ-induced invasion and cell-cycle regulation via Lys63-linked polyubiquitination of Lys178 in TGFβ type I receptor.Cell Cycle. 2015;14(4):554-65. doi: 10.4161/15384101.2014.990302. Cell Cycle. 2015. PMID: 25622187 Free PMC article.
-
The Ubiquitin Tale: Current Strategies and Future Challenges.ACS Pharmacol Transl Sci. 2024 Sep 4;7(9):2573-2587. doi: 10.1021/acsptsci.4c00278. eCollection 2024 Sep 13. ACS Pharmacol Transl Sci. 2024. PMID: 39296276 Free PMC article. Review.
-
Structural Insight into Anaphase Promoting Complex 3 Structure and Docking with a Natural Inhibitory Compound.Adv Biomed Res. 2017 Mar 7;6:26. doi: 10.4103/2277-9175.201683. eCollection 2017. Adv Biomed Res. 2017. PMID: 28401073 Free PMC article.
-
β2M Signals Monocytes Through Non-Canonical TGFβ Receptor Signal Transduction.Circ Res. 2021 Mar 5;128(5):655-669. doi: 10.1161/CIRCRESAHA.120.317119. Epub 2021 Jan 29. Circ Res. 2021. PMID: 33508948 Free PMC article.
References
-
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–84. - PubMed
-
- Mu Y, Gudey SK, Landstrom M. Non-Smad signaling pathways. Cell Tissue Res. 2012;347:11–20. - PubMed
-
- Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–63. - PubMed
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
