Insight into the cooperation of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) at the blood-brain barrier: a case study examining sorafenib efflux clearance
- PMID: 22335402
- PMCID: PMC3296237
- DOI: 10.1021/mp200465c
Insight into the cooperation of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) at the blood-brain barrier: a case study examining sorafenib efflux clearance
Abstract
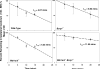
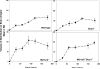
The ATP-binding cassette transporters P-glycoprotein and breast cancer resistance protein have been shown to be critical determinants limiting drug transport across the BBB into the brain. Several therapeutic agents have been shown to be substrates for these two transporters, and as a result they have limited distribution to the brain. Recently, it has been shown that these two drug transporters cooperate at the BBB and brain penetration of dual substrates increases significantly only when both are absent, e.g., in the Mdr1a/1b(-/-)Bcrp1(-/-) mice. The present study uses the brain penetration of sorafenib to investigate these findings and attempts to explain the mechanistic basis of this cooperation with a simple theory based on affinity and capacity dependent carrier-mediated transport. The brain efflux index method, combined with the organotypic brain slices, was used to determine the net contribution of P-gp and BCRP to the total clearance of sorafenib out of the brain and show that its efflux at the BBB is mediated primarily by BCRP. Sorafenib clearance out of the brain decreased 2-fold in the Bcrp1(-/-) mice and 2.5-fold in the Mdr1a/1b(-/-)Bcrp1(-/-) mice. Clearance out of brain when P-gp was absent did not change significantly compared to wild-type. We also investigated the expression of P-gp and BCRP in the genetic knockout animals and saw no differences in either P-gp or BCRP in the transporter deficient mice compared to the wild-type mice. In conclusion, this study explains the cooperation of P-gp and BCRP by analysis of the efflux clearance of sorafenib and correlating it to the "mechanisms" that determine the clearance, i.e., affinity and capacity.
Figures

References
-
- Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, Pardridge W, Rosenberg GA, Smith Q, Drewes LR. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7(1):84–96. - PubMed
-
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O'Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12(3):169–82. - PMC - PubMed
-
- Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55(1):3–29. - PubMed
-
- de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13(21):6440–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

