Intramuscular versus intravenous therapy for prehospital status epilepticus
- PMID: 22335736
- PMCID: PMC3307101
- DOI: 10.1056/NEJMoa1107494
Intramuscular versus intravenous therapy for prehospital status epilepticus
Abstract
Background: Early termination of prolonged seizures with intravenous administration of benzodiazepines improves outcomes. For faster and more reliable administration, paramedics increasingly use an intramuscular route.
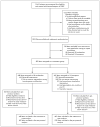
Methods: This double-blind, randomized, noninferiority trial compared the efficacy of intramuscular midazolam with that of intravenous lorazepam for children and adults in status epilepticus treated by paramedics. Subjects whose convulsions had persisted for more than 5 minutes and who were still convulsing after paramedics arrived were given the study medication by either intramuscular autoinjector or intravenous infusion. The primary outcome was absence of seizures at the time of arrival in the emergency department without the need for rescue therapy. Secondary outcomes included endotracheal intubation, recurrent seizures, and timing of treatment relative to the cessation of convulsive seizures. This trial tested the hypothesis that intramuscular midazolam was noninferior to intravenous lorazepam by a margin of 10 percentage points.
Results: At the time of arrival in the emergency department, seizures were absent without rescue therapy in 329 of 448 subjects (73.4%) in the intramuscular-midazolam group and in 282 of 445 (63.4%) in the intravenous-lorazepam group (absolute difference, 10 percentage points; 95% confidence interval, 4.0 to 16.1; P<0.001 for both noninferiority and superiority). The two treatment groups were similar with respect to need for endotracheal intubation (14.1% of subjects with intramuscular midazolam and 14.4% with intravenous lorazepam) and recurrence of seizures (11.4% and 10.6%, respectively). Among subjects whose seizures ceased before arrival in the emergency department, the median times to active treatment were 1.2 minutes in the intramuscular-midazolam group and 4.8 minutes in the intravenous-lorazepam group, with corresponding median times from active treatment to cessation of convulsions of 3.3 minutes and 1.6 minutes. Adverse-event rates were similar in the two groups.
Conclusions: For subjects in status epilepticus, intramuscular midazolam is at least as safe and effective as intravenous lorazepam for prehospital seizure cessation. (Funded by the National Institute of Neurological Disorders and Stroke and others; ClinicalTrials.gov number, ClinicalTrials.gov NCT00809146.).
Figures


Comment in
-
Intramuscular versus intravenous benzodiazepines for prehospital treatment of status epilepticus.N Engl J Med. 2012 Feb 16;366(7):659-60. doi: 10.1056/NEJMe1114206. N Engl J Med. 2012. PMID: 22335744 No abstract available.
-
Intramuscular versus intravenous benzodiazepines for status epilepticus.N Engl J Med. 2012 May 17;366(20):1943; author reply 1944. doi: 10.1056/NEJMc1203428#SA1. N Engl J Med. 2012. PMID: 22591303 No abstract available.
-
Intramuscular versus intravenous benzodiazepines for status epilepticus.N Engl J Med. 2012 May 17;366(20):1943-4; author reply 1944. doi: 10.1056/NEJMc1203428. N Engl J Med. 2012. PMID: 22591304 No abstract available.
-
Management of status epilepticus.J Neurol. 2012 Oct;259(10):2261-3. doi: 10.1007/s00415-012-6673-5. J Neurol. 2012. PMID: 23001523 No abstract available.
References
-
- Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345:631–7. [Erratum, N Engl J Med 2001;345:1860.] - PubMed
-
- Warden CR, Frederick C. Midazolam and diazepam for pediatric seizures in the prehospital setting. Prehosp Emerg Care. 2006;10:463–7. - PubMed
-
- Gottwald MD, Akers LC, Liu PK, et al. Prehospital stability of diazepam and lorazepam. Am J Emerg Med. 1999;17:333–7. - PubMed
-
- Exception from informed consent requirements for emergency research: Code of Federal Regulations. Washington, DC: Government Printing Office; ( http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr...)
-
- Lowenstein DH, Alldredge BK, Allen F, et al. The Prehospital Treatment of Status Epilepticus (PHTSE) study: design and methodology. Control Clin Trials. 2001;22:290–309. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
