Paraneoplastic thrombocytosis in ovarian cancer
- PMID: 22335738
- PMCID: PMC3296780
- DOI: 10.1056/NEJMoa1110352
Paraneoplastic thrombocytosis in ovarian cancer
Erratum in
- N Engl J Med. 2012 Nov;367(18):1768. Lamkin, Donald [added]
Abstract
Background: The mechanisms of paraneoplastic thrombocytosis in ovarian cancer and the role that platelets play in abetting cancer growth are unclear.
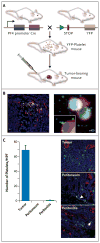
Methods: We analyzed clinical data on 619 patients with epithelial ovarian cancer to test associations between platelet counts and disease outcome. Human samples and mouse models of epithelial ovarian cancer were used to explore the underlying mechanisms of paraneoplastic thrombocytosis. The effects of platelets on tumor growth and angiogenesis were ascertained.
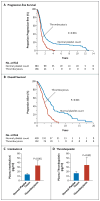
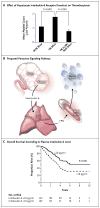
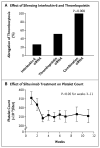
Results: Thrombocytosis was significantly associated with advanced disease and shortened survival. Plasma levels of thrombopoietin and interleukin-6 were significantly elevated in patients who had thrombocytosis as compared with those who did not. In mouse models, increased hepatic thrombopoietin synthesis in response to tumor-derived interleukin-6 was an underlying mechanism of paraneoplastic thrombocytosis. Tumor-derived interleukin-6 and hepatic thrombopoietin were also linked to thrombocytosis in patients. Silencing thrombopoietin and interleukin-6 abrogated thrombocytosis in tumor-bearing mice. Anti-interleukin-6 antibody treatment significantly reduced platelet counts in tumor-bearing mice and in patients with epithelial ovarian cancer. In addition, neutralizing interleukin-6 significantly enhanced the therapeutic efficacy of paclitaxel in mouse models of epithelial ovarian cancer. The use of an antiplatelet antibody to halve platelet counts in tumor-bearing mice significantly reduced tumor growth and angiogenesis.
Conclusions: These findings support the existence of a paracrine circuit wherein increased production of thrombopoietic cytokines in tumor and host tissue leads to paraneoplastic thrombocytosis, which fuels tumor growth. We speculate that countering paraneoplastic thrombocytosis either directly or indirectly by targeting these cytokines may have therapeutic potential. (Funded by the National Cancer Institute and others.).
Conflict of interest statement
Dr. Stone reports being listed as one of the inventors on a U.S. patent entitled “Use of Selective Adenosine A1 Receptor Allosteric Enhancers to Manipulate Angiogenesis.” No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Paraneoplastic thrombocytosis in ovarian cancer.N Engl J Med. 2012 May 10;366(19):1840; author reply 1840. doi: 10.1056/NEJMc1203095. N Engl J Med. 2012. PMID: 22571210 No abstract available.
References
-
- Leslie M. Cell biology: beyond clotting: the powers of platelets. Science. 2010;328:562–4. - PubMed
-
- Trousseau A, Bazire V, Cormack J. Lectures on clinical medicine, delivered at the Hôtel-Dieu, Paris. London: R. Hardwicke; 1867.
-
- Riess L. Zur pathologischen anatomie des blutes. Arch Anat Physiol Wissensch Med. 1872;39:237–49.
-
- Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964;114:497–500. - PubMed
-
- Borsig L. The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther. 2008;8:1247–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- CA016672/CA/NCI NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- R01 CA104825/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- R01 CA128797/CA/NCI NIH HHS/United States
- R01 CA140933/CA/NCI NIH HHS/United States
- T32 CA009666/CA/NCI NIH HHS/United States
- CA140933/CA/NCI NIH HHS/United States
- T32 CA101642/CA/NCI NIH HHS/United States
- G0501974/MRC_/Medical Research Council/United Kingdom
- CA109298/CA/NCI NIH HHS/United States
- CA128797/CA/NCI NIH HHS/United States
- R01 CA109298/CA/NCI NIH HHS/United States
- RC2GM092599/GM/NIGMS NIH HHS/United States
- U54 CA151668/CA/NCI NIH HHS/United States
- RC2 GM092599/GM/NIGMS NIH HHS/United States
- G0601891/MRC_/Medical Research Council/United Kingdom
- R01CA104825/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
