Challenging the role of adaptive immunity in neurotrauma: Rag1(-/-) mice lacking mature B and T cells do not show neuroprotection after closed head injury
- PMID: 22335783
- PMCID: PMC3325549
- DOI: 10.1089/neu.2011.2169
Challenging the role of adaptive immunity in neurotrauma: Rag1(-/-) mice lacking mature B and T cells do not show neuroprotection after closed head injury
Abstract
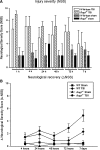
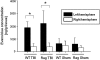
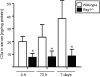
The role of adaptive immunity in contributing to post-traumatic neuroinflammation and neuropathology after head injury remains largely unexplored. The present study was designed to investigate the pathophysiological sequelae of closed head injury in Rag1(-/-) mice devoid of mature B and T lymphocytes. C57BL/6 wild-type and Rag1(-/-) mice were subjected to experimental closed head injury, using a standardized weight-drop device. Outcome parameters consisted of neurological scoring, quantification of blood-brain barrier (BBB) function, measurement of inflammatory markers and mediators of apoptosis in serum and brain tissue, and assessment of neuronal cell death, astrogliosis, and tissue destruction. There was no difference between wild-type and Rag1(-/-) mice with regard to injury severity and neurological impairment for up to 7 days after head injury. The extent of BBB dysfunction was in a similar range for both groups. Quantification of complement activation fragments in serum revealed significantly attenuated C3a levels in Rag1(-/-) mice compared to wild-type animals. In contrast, the levels of pro- and anti-inflammatory cytokines and pro-apoptotic and anti-apoptotic mediators remained in a similar range for both groups, and the histological analysis of brain sections did not reveal a difference in reactive astrogliosis, tissue destruction, and neuronal cell death in Rag1(-/-) compared to wild-type mice. These findings suggest that adaptive immunity is not of crucial importance for initiating and sustaining the inflammatory neuropathology after closed head injury. The attenuated extent of post-traumatic complement activation seen in Rag1(-/-) mice implies a cross-talk between innate and adaptive immune responses, which requires further investigation in future studies.
Figures



References
-
- Austen W.G., Jr. Zhang M. Chan R. Friend D. Hechtman H.B. Carroll M.C. Moore F.D., Jr. Murine hindlimb reperfusion injury can be initiated by a self-reactive monoclonal IgM. Surgery. 2004;136:401–406. - PubMed
-
- Bechmann I. Galea I. Perry V.H. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

