A comparison of optical and electrophysiological methods for recording retinal ganglion cells during electrical stimulation
- PMID: 22335809
- PMCID: PMC3707149
- DOI: 10.3109/02713683.2011.652756
A comparison of optical and electrophysiological methods for recording retinal ganglion cells during electrical stimulation
Abstract
Purpose/aim: To compare the efficacy of optical techniques with electrophysiological recordings for mapping retinal activity in response to electrical stimulation.
Materials and methods: Whole cell patch clamp, Ca(2+) imaging (Fluo-4-AM), and Na(+) imaging (CoroNa Green-AM) techniques were used to detect responses of neurons from mouse and salamander retina to electrical stimulation.
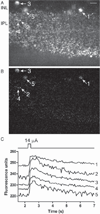
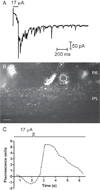
Results: Synaptic currents were observed in ≥23% of retinal ganglion cells (RGCs), indicating presynaptic Ca(2+) increases in the inner plexiform layer (IPL). Modest depolarization with 20-30 mM K(+) consistently evoked Ca(2+) responses measured with Fluo4, but Ca(2+) responses were almost never evoked by epiretinal stimulation. In salamander retina, responses were seen in the inner nuclear layer (INL) and IPL. In mouse retina, responses were also sometimes seen in the outer pexiform layer (OPL). OPL responses showed a longer latency than IPL responses, suggesting that outer retinal circuits do not trigger synaptic responses of RGCs. Simultaneous Ca(2+) imaging and electrophysiological recording of synaptic currents confirmed that Fluo4-loaded retinas remained responsive to stimulation. Epiretinal stimulation evoked action potentials in ≥67% of RGCs. CoroNa Green detected Na(+) changes stimulated by 20 mM K(+), but epiretinal stimulation did not evoke detectable Na(+) responses. Simultaneous imaging and electrophysiological recording confirmed the health of CoroNa Green-loaded retinas. We confirmed stimulation efficacy by simultaneously recording Na(+) changes and electrophysiological responses.
Conclusions: These data demonstrate that electrophysiological recordings show greater sensitivity than Na(+) or Ca(2+) imaging in response to electrical stimulation. The paucity of Ca(2+) responses is consistent with limited risk for Ca(2+)-mediated cell damage during electrical stimulation.
Figures






Similar articles
-
Inner and outer retinal mechanisms engaged by epiretinal stimulation in normal and rd mice.Vis Neurosci. 2011 Mar;28(2):145-54. doi: 10.1017/S0952523810000489. Vis Neurosci. 2011. PMID: 21463541 Free PMC article.
-
Alpha2 adrenergic receptor-mediated modulation of cytosolic Ca++ signals at the inner plexiform layer of the rat retina.Invest Ophthalmol Vis Sci. 2007 Mar;48(3):1410-5. doi: 10.1167/iovs.06-0890. Invest Ophthalmol Vis Sci. 2007. PMID: 17325190
-
Inner retinal mechanisms engaged by retinal electrical stimulation.Invest Ophthalmol Vis Sci. 2006 Jun;47(6):2606-12. doi: 10.1167/iovs.05-1093. Invest Ophthalmol Vis Sci. 2006. PMID: 16723477 Free PMC article.
-
Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina.J Physiol. 1987 Apr;385:361-91. doi: 10.1113/jphysiol.1987.sp016497. J Physiol. 1987. PMID: 2443669 Free PMC article. Review.
-
Combining calcium imaging with other optical techniques.Cold Spring Harb Protoc. 2013 Dec 1;2013(12):1125-31. doi: 10.1101/pdb.top066167. Cold Spring Harb Protoc. 2013. PMID: 24298025 Free PMC article. Review.
Cited by
-
Polymer-free optode nanosensors for dynamic, reversible, and ratiometric sodium imaging in the physiological range.Sci Rep. 2013 Nov 28;3:3366. doi: 10.1038/srep03366. Sci Rep. 2013. PMID: 24284431 Free PMC article.
-
Simultaneous whole-cell recordings from photoreceptors and second-order neurons in an amphibian retinal slice preparation.J Vis Exp. 2013 Jun 1;(76):50007. doi: 10.3791/50007. J Vis Exp. 2013. PMID: 23770753 Free PMC article.
-
From retina to motoneurons: A substrate for visuomotor transformation in salamanders.J Comp Neurol. 2022 Oct;530(14):2518-2536. doi: 10.1002/cne.25348. Epub 2022 Jun 3. J Comp Neurol. 2022. PMID: 35662021 Free PMC article.
References
-
- Phelan JK, Bok D. A brief review of retinitis pigmentosa and the identified retinitis pigmentosa genes. Mol Vis. 2000;6:116–124. - PubMed
-
- Stone JL, Barlow WE, Humayun MS, de Juan E, Jr., Milam AH. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol. 1992;110:1634–1639. - PubMed
-
- Fried SI, Hsueh HA, Werblin FS. A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation. J Neurophysiol. 2006;95:970–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
