Discoidin domain receptors regulate the migration of primary human lung fibroblasts through collagen matrices
- PMID: 22336030
- PMCID: PMC3298810
- DOI: 10.1186/1755-1536-5-3
Discoidin domain receptors regulate the migration of primary human lung fibroblasts through collagen matrices
Abstract
Background: The two discoidin domain receptors (DDRs), DDR1 and DDR2 are receptor tyrosine kinases (RTKs) with the unique ability among RTKs to respond to collagen. We have previously shown that collagen I induces DDR1 and matrix metalloproteinase (MMP)-10 expression through DDR2 activation and a Janus kinase (JAK)2 and extracellular signal-regulated kinase (ERK)1/2-mediated mechanism in primary human lung fibroblasts suggesting that these signaling pathways play a role in fibroblast function. Fibroblasts can traverse basement membrane barriers during development, wound healing and pathological conditions such as cancer and fibrosis by activating tissue-invasive programs, the identity of which remain largely undefined. In the present work, we investigated the role of DDRs and DDR-associated signal transduction in these processes.
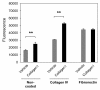
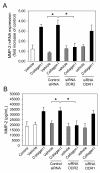
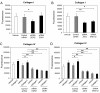
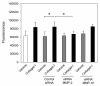
Results: Transwell migration experiments showed that normal human lung fibroblast (NHLF) transmigration through collagen I-coated inserts is mediated by DDR2 and the DDR2-associated signaling kinases JAK2 and ERK1/2, but not DDR1. Additionally, experiments with specific small interfering (si)RNAs revealed that collagen I-induced expression of MMP-10 and MMP-2 is DDR2 but not DDR1 dependent in NHLFs. Our data showed that collagen I increases NHLF migration through collagen IV, the main component of basement membranes. Furthermore, basal and collagen I-induced NHLF migration through collagen IV-coated inserts was both DDR2 and DDR1 dependent. Finally, DDR2, but not DDR1 was shown to be involved in fibroblast proliferation.
Conclusions: Our results suggest a mechanism by which the presence of collagen I in situations of excessive matrix deposition could induce fibroblast migration through basement membranes through DDR2 activation and subsequent DDR1 and MMP-2 gene expression. This work provides new insights into the role of DDRs in fibroblast function.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

