Preventing Pseudomonas aeruginosa and Chromobacterium violaceum infections by anti-adhesion-active components of edible seeds
- PMID: 22336073
- PMCID: PMC3361497
- DOI: 10.1186/1475-2891-11-10
Preventing Pseudomonas aeruginosa and Chromobacterium violaceum infections by anti-adhesion-active components of edible seeds
Abstract
Background: Pseudomonas aeruginosa adhesion to animal/human cells for infection establishment involves adhesive proteins, including its galactose- and fucose-binding lectins PA-IL (LecA) and PA-IIL (LecB). The lectin binding to the target-cell receptors may be blocked by compatible glycans that compete with those of the receptors, functioning as anti-adhesion glycodecoys. The anti-adhesion treatment is of the utmost importance for abrogating devastating antibiotic-resistant P. aeruginosa infections in immunodeficient and cystic fibrosis (CF) patients. This strategy functions in nature in protecting embryos and neonates. We have shown that PA-IL, PA-IIL, and also CV-IIL (a PA-IIL homolog produced in the related pathogen Chromobacterium violaceum) are highly useful for revealing natural glycodecoys that surround embryos in diverse avian eggs and are supplied to neonates in milks and royal jelly. In the present study, these lectins were used as probes to search for seed embryo-protecting glycodecoys.
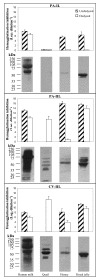
Methods: The lectin-blocking glycodecoy activities were shown by the hemagglutination-inhibition test. Lectin-binding glycoproteins were detected by Western blotting with peroxidase-labeled lectins.
Results: The present work reports the finding - by using PA-IL, PA-IIL, and CV-IIL - of rich glycodecoy activities of low (< 10 KDa) and high MW (> 10 kDa) compounds (including glycoproteins) in extracts of cashew, cocoa, coffee, pumpkin, and tomato seeds, resembling those of avian egg whites, mammal milks, and royal jelly.
Conclusions: Edible seed extracts possess lectin-blocking glycodecoys that might protect their embryos from infections and also might be useful for hampering human and animal infections.
Figures


Similar articles
-
Blocking of Pseudomonas aeruginosa and Chromobacterium violaceum lectins by diverse mammalian milks.J Dairy Sci. 2010 Feb;93(2):473-82. doi: 10.3168/jds.2009-2381. J Dairy Sci. 2010. PMID: 20105519
-
Preventing Ralstonia solanacearum adhesion with glycans from cashew, cocoa, coffee, pumpkin, and tomato seed extract.Can J Microbiol. 2012 Jul;58(7):856-62. doi: 10.1139/w2012-062. Epub 2012 Jun 19. Can J Microbiol. 2012. PMID: 22712584
-
Blocking of Pseudomonas aeruginosa and Ralstonia solanacearum Lectins by plant and microbial branched polysaccharides used as food additives.J Agric Food Chem. 2009 Aug 12;57(15):6908-13. doi: 10.1021/jf900631j. J Agric Food Chem. 2009. PMID: 19572540
-
Structures of the lectins from Pseudomonas aeruginosa: insight into the molecular basis for host glycan recognition.Microbes Infect. 2004 Feb;6(2):221-8. doi: 10.1016/j.micinf.2003.10.016. Microbes Infect. 2004. PMID: 15049333 Review.
-
Adhesins and receptors of Pseudomonas aeruginosa associated with infection of the respiratory tract.Microb Pathog. 1992 Oct;13(4):251-60. doi: 10.1016/0882-4010(92)90035-m. Microb Pathog. 1992. PMID: 1363702 Review.
Cited by
-
Royal Jelly Inhibits Pseudomonas aeruginosa Adherence and Reduces Excessive Inflammatory Responses in Human Epithelial Cells.Biomed Res Int. 2017;2017:3191752. doi: 10.1155/2017/3191752. Epub 2017 Sep 17. Biomed Res Int. 2017. PMID: 29075644 Free PMC article.
-
Carbohydrate coating reduces adhesion of biofilm-forming Bacillus subtilis to gold surfaces.Appl Environ Microbiol. 2014 Oct;80(19):5911-7. doi: 10.1128/AEM.01600-14. Epub 2014 Jul 18. Appl Environ Microbiol. 2014. PMID: 25038098 Free PMC article.
-
Novel Treatment Strategies for Biofilm-Based Infections.Drugs. 2019 Oct;79(15):1635-1655. doi: 10.1007/s40265-019-01184-z. Drugs. 2019. PMID: 31468316 Review.
-
Pseudomonas Aeruginosa Lectins As Targets for Novel Antibacterials.Acta Naturae. 2015 Apr-Jun;7(2):29-41. Acta Naturae. 2015. PMID: 26085942 Free PMC article.
References
-
- Gilboa-Garber N. Pseudomonas aeruginosa lectins. Methods Enzymol. 1982;83:378–385. - PubMed
-
- Zinger-Yosovich K, Sudakevitz D, Imberty A, Garber NC, Gilboa-Garber N. Production and properties of the native Chromobacterium violaceum fucose-binding lectin (CV-IIL) compared to homologous lectins of Pseudomonas aeruginosa (PA-IIL) and Ralstonia solanacearum (RS-IIL) Microbiology. 2006;152:457–463. doi: 10.1099/mic.0.28500-0. - DOI - PubMed
-
- Gilboa-Garber N, Sudakevitz D, Sheffi M, Sela R, Levene C. PA-I and PA-II lectin interactions with the ABO(H) and P blood group glycosphingolipid antigens may contribute to the broad spectrum adherence of Pseudomonas aeruginosa to human tissues in secondary infections. Glycoconj J. 1994;11:414–417. doi: 10.1007/BF00731276. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

