Sexual and asexual oogenesis require the expression of unique and shared sets of genes in the insect Acyrthosiphon pisum
- PMID: 22336141
- PMCID: PMC3313892
- DOI: 10.1186/1471-2164-13-76
Sexual and asexual oogenesis require the expression of unique and shared sets of genes in the insect Acyrthosiphon pisum
Abstract
Background: Although sexual reproduction is dominant within eukaryotes, asexual reproduction is widespread and has evolved independently as a derived trait in almost all major taxa. How asexuality evolved in sexual organisms is unclear. Aphids, such as Acyrthosiphon pisum, alternate between asexual and sexual reproductive means, as the production of parthenogenetic viviparous females or sexual oviparous females and males varies in response to seasonal photoperiodism. Consequently, sexual and asexual development in aphids can be analyzed simultaneously in genetically identical individuals.
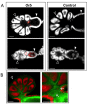
Results: We compared the transcriptomes of aphid embryos in the stages of development during which the trajectory of oogenesis is determined for producing sexual or asexual gametes. This study design aimed at identifying genes involved in the onset of the divergent mechanisms that result in the sexual or asexual phenotype. We detected 33 genes that were differentially transcribed in sexual and asexual embryos. Functional annotation by gene ontology (GO) showed a biological signature of oogenesis, cell cycle regulation, epigenetic regulation and RNA maturation. In situ hybridizations demonstrated that 16 of the differentially-transcribed genes were specifically expressed in germ cells and/or oocytes of asexual and/or sexual ovaries, and therefore may contribute to aphid oogenesis. We categorized these 16 genes by their transcription patterns in the two types of ovaries; they were: i) expressed during sexual and asexual oogenesis; ii) expressed during sexual and asexual oogenesis but with different localizations; or iii) expressed only during sexual or asexual oogenesis.
Conclusions: Our results show that asexual and sexual oogenesis in aphids share common genetic programs but diverge by adapting specificities in their respective gene expression profiles in germ cells and oocytes.
Figures



Similar articles
-
Gene expression analysis of parthenogenetic embryonic development of the pea aphid, Acyrthosiphon pisum, suggests that aphid parthenogenesis evolved from meiotic oogenesis.PLoS One. 2014 Dec 12;9(12):e115099. doi: 10.1371/journal.pone.0115099. eCollection 2014. PLoS One. 2014. PMID: 25501006 Free PMC article.
-
The pea aphid (Acyrthosiphon pisum) genome encodes two divergent early developmental programs.Dev Biol. 2013 May 1;377(1):262-74. doi: 10.1016/j.ydbio.2013.01.036. Epub 2013 Feb 13. Dev Biol. 2013. PMID: 23416037
-
Anterior development in the parthenogenetic and viviparous form of the pea aphid, Acyrthosiphon pisum: hunchback and orthodenticle expression.Insect Mol Biol. 2010 Mar;19 Suppl 2:75-85. doi: 10.1111/j.1365-2583.2009.00940.x. Insect Mol Biol. 2010. PMID: 20482641
-
Germline specification and axis determination in viviparous and oviparous pea aphids: conserved and divergent features.Dev Genes Evol. 2022 Aug;232(2-4):51-65. doi: 10.1007/s00427-022-00690-7. Epub 2022 Jun 9. Dev Genes Evol. 2022. PMID: 35678925 Free PMC article. Review.
-
Shifting from clonal to sexual reproduction in aphids: physiological and developmental aspects.Biol Cell. 2008 Aug;100(8):441-51. doi: 10.1042/BC20070135. Biol Cell. 2008. PMID: 18627352 Review.
Cited by
-
The reproductive system of the male and oviparous female of a model organism-the pea aphid, Acyrthosiphon pisum (Hemiptera, Aphididae).PeerJ. 2019 Sep 2;7:e7573. doi: 10.7717/peerj.7573. eCollection 2019. PeerJ. 2019. PMID: 31534847 Free PMC article.
-
Contrasting Evolutionary Patterns Between Sexual and Asexual Lineages in a Genomic Region Linked to Reproductive Mode Variation in the pea aphid.Genome Biol Evol. 2023 Sep 4;15(9):evad168. doi: 10.1093/gbe/evad168. Genome Biol Evol. 2023. PMID: 37717171 Free PMC article.
-
Comparative transcriptional analysis of asexual and sexual morphs reveals possible mechanisms in reproductive polyphenism of the cotton aphid.PLoS One. 2014 Jun 10;9(6):e99506. doi: 10.1371/journal.pone.0099506. eCollection 2014. PLoS One. 2014. PMID: 24915491 Free PMC article.
-
Transcriptomic basis of sex loss in the pea aphid.BMC Genomics. 2024 Feb 21;25(1):202. doi: 10.1186/s12864-023-09776-6. BMC Genomics. 2024. PMID: 38383295 Free PMC article.
-
The Effects of Different Diets and Transgenerational Stress on Acyrthosiphon pisum Development.Insects. 2019 Aug 21;10(9):260. doi: 10.3390/insects10090260. Insects. 2019. PMID: 31438654 Free PMC article.
References
-
- Schön I, Martens K, van Dijk P, editor. Lost sex, the evolutionary biology of parthenogenesis. Springer; 2009.
-
- Simon JC, Delmotte F, Rispe C, Crease T. Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals. Biol J Linn Soc. 2003;79:151–163. doi: 10.1046/j.1095-8312.2003.00175.x. - DOI
-
- Hörandl E. In: Lost sex, the evolutionary biology of parthenogenesis. Schön I, Martens, K., and van Dijk, P, editor. Springer; 2009. Geographical Parthenogenesis: Opportunities for Asexuality; pp. 161–186.
-
- Dixon AFG. Aphid ecology. 2. Chapman & Hall; 1998.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

