Putative sequences on Ro60 three-dimensional structure accessible for 4-hydroxy-2-nonenal (HNE) modification compared to in vitro HNE modification of Ro60 sequences
- PMID: 22336572
- PMCID: PMC8459514
- DOI: 10.1016/j.molimm.2011.12.010
Putative sequences on Ro60 three-dimensional structure accessible for 4-hydroxy-2-nonenal (HNE) modification compared to in vitro HNE modification of Ro60 sequences
Abstract
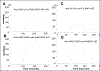
We have previously reported accelerated acquisition of new autoreactivity upon immunization with 4-hydroxy-2-nonenal (HNE)-modified Ro60, as well as differential induction of lupus or Sjögren's syndrome by immunization with Ro60 containing varying amounts of HNE. Since the number of HNE molecules on Ro60 appears to be important, we hypothesized that specific sequences on Ro60 are targets for HNE-modification. Using surface plasmon resonance (SPR) we have also shown intramolecular protein-protein interaction between Ro60 and Ro multiple antigenic peptides (MAPs). We also hypothesized that intramolecular protein-protein interaction would be abolished by HNE-modification. To test this hypotheses we investigated (a) the epitopes of Ro60, using 19 Ro MAPs in an in vitro assay (involving HNE-modification of MAPs following immobilization on ELISA plates) to identify targets of HNE modification on Ro60 and (b) the protein-protein interaction between unmodified Ro60 MAPs, immobilized on the sensor surface of BIAcore, and unmodified Ro60 or HNE-modified Ro60 using SPR. New data obtained with SPR strengthens our earlier observation that immunization with HNE-Ro60 induces a stronger response. Unmodified Ro60 bound to several Ro60 MAPs through protein-protein interaction analyzed using SPR. This interaction was totally abrogated using HNE-modified Ro60 suggesting that sequences on Ro had become modified with HNE. When 19 Ro60 MAPs were modified in vitro with HNE, it was found that 10/19 MAPs significantly bound HNE covalently (p<0.001 compared to MAPs binding HNE poorly). The amino acid sequences 126-137, 166-272 and 401-495 on Ro60 were strongly HNE modified. Using computational model system based on the recently published crystal structure for Ro60 enabled us to identify regions on the Ro60 molecule represented by the HNE-modified Ro MAPs, which are part of the exposed tertiary structure of the Ro60 protein.
Copyright © 2012. Published by Elsevier Ltd.
Figures





References
-
- Di Domenico F, Sultana R, Tiu GF, Scheff NN, Perluigi M, Cini C, Butterfield DA, 2010. Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Research 1333, 72–81. - PMC - PubMed
-
- D’souza A, Kurien BT, Rodgers R, Shenoi J, Kurono S, Matsumoto H, Hensley K, Nath SK, Scofield RH, 2008. Detection of catalase as a major protein target of the lipid peroxidation product 4-HNE and the lack of its genetic association as a risk factor in SLE. BMC Medical Genetics. 20089, 62–69. - PMC - PubMed
-
- Dickey WD, Egmond JEV, Hardgrave KL, Harley JB, 1993. Presence of anti-La (SS-B) is associated with binding to the 13-kD carboxyl terminus of 60-kD Ro (SS-A) in systemic lupus erythematosus. Journal of Investigative Dermatology 100, 412–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

