Growth hormone for children with chronic kidney disease
- PMID: 22336787
- PMCID: PMC6599873
- DOI: 10.1002/14651858.CD003264.pub3
Growth hormone for children with chronic kidney disease
Abstract
Background: Growth retardation is a common complication of chronic kidney disease (CKD) in children and is of concern to families. Recombinant human growth hormone (rhGH) treatment has been used to help short children with CKD attain a height more in keeping with their age group. However there are concerns about the long-term benefits of rhGH in significantly improving adult height as well as concerns about potential adverse effects (deterioration in native kidney function, increased acute rejection in kidney transplant recipients, benign intracranial hypertension).
Objectives: To evaluate the benefits and harms of rhGH treatment in children with CKD.
Search methods: Randomised controlled trials (RCTs) were identified from the Cochrane Renal Group's Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 12, 2011), MEDLINE (from 1966), EMBASE (from 1980), article reference lists and through contact with local and international experts in the field.Date of last search: December 29, 2011
Selection criteria: RCTs were included if they were carried out in children aged zero to 18 years, diagnosed with CKD, who were pre-dialysis, on dialysis or post-transplant; if they compared rhGH treatment with placebo/no treatment or two doses of rhGH treatments; and if they included height outcomes.
Data collection and analysis: Two authors independently assessed studies for risk of bias and extracted data from eligible studies. Data was pooled using a random effects model with calculation of mean difference (MD) for continuous outcomes with 95% confidence intervals (CI).
Main results: Sixteen studies (enrolling 809 children) were identified. Risk of bias assessment indicated that study quality was poor or poorly reported with only four and five studies respectively reporting adequate allocation concealment or blinding of study participants and investigators. Treatment with rhGH (28 IU/m²/wk) compared with placebo or no specific therapy resulted in a significant increase in height standard deviation score (HSDS) at one year (8 studies, 391 children: MD 0.82, 95% CI 0.56 to 1.07), and a significant increase in height velocity at six months (2 studies, 27 children: MD 2.85 cm/6 mo, 95% CI 2.22 to 3.48) and one year (7 studies, 287 children: MD 3.88 cm/y, 95% CI 3.32 to 4.44). Height velocity, though reduced, remained significantly greater than untreated children during the second year of therapy (1 study, 82 children: MD 2.30 cm/y, 95% CI 1.39 to 3.21). Compared to the 14 IU/m²/wk group, there was a 1.18 cm/y increase in height velocity in the 28 IU/m²/wk group (3 studies, 150 children: 1.18 cm/y, 95% CI 0.52 to 1.84) . The frequency of reported side effects of rhGH was generally similar to that of the control group.
Authors' conclusions: One year of 28 IU/m²/wk rhGH in children with CKD resulted in a 3.88 cm increase in height velocity above that of untreated patients. Studies were too short to determine if continuing treatment resulted in an increase in final adult height.
Conflict of interest statement
None known.
Figures

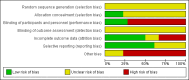
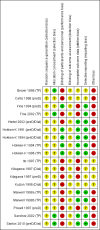


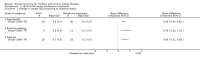






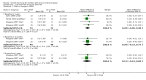


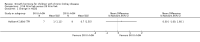
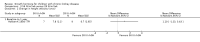
Update of
-
Growth hormone for children with chronic kidney disease.Cochrane Database Syst Rev. 2006 Jul 19;(3):CD003264. doi: 10.1002/14651858.CD003264.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003264. doi: 10.1002/14651858.CD003264.pub3. PMID: 16856001 Updated.
References
References to studies included in this review
Broyer 1996 (TP) {published data only}
-
- Broyer M. Results and side‐effects of treating children with growth hormone after kidney transplantation ‐ a preliminary report. Pharmacia & Upjohn Study Group. Acta Paediatrica Supplement 1996;417:76‐9. [MEDLINE: ] - PubMed
-
- Broyer M, Guest G, Crosnier H, Berard E, on behalf of the French Society for Pediatric Nephrology. A multicentric open randomized controlled study of rhGH therapy in short children after kidney transplantation [abstract]. Journal of the American Society of Nephrology 1995;6(3):1075.
-
- Broyer M, on behalf of the study group. A multi‐centre open, randomized, controlled study evaluating the effect of growth hormone therapy in short children after renal transplantation [abstract]. Pediatric Nephrology 1995;9(6):C49.
-
- Guest G, Berard E, Crosnier H, Chevallier T, Rappaport R, Broyer M. Effects of growth hormone in short children after renal transplantation. Pediatric Nephrology 1998;12(6):437‐46. [MEDLINE: ] - PubMed
Callis 1996 (preD) {published data only}
-
- Callis LL, Valle CJ, Garcia L, Garcia Nieto V, Gonzalez Diaz JP, Heras M, et al. Randomized open multicentre study to assess treatment with recombinant human growth hormone in children with low height and chronic renal insufficiency [abstract]. Anales Espanoles de Pediatria 1996;45(3):328.
Fine 1994 (preD) {published data only}
-
- Fine RN, Attie KM, Kuntze J, Brown DF, Kohaut EC. Recombinant human growth hormone in infants and young children with chronic renal insufficiency. Genentech Collaborative Study Group. Pediatric Nephrology 1995;9:451‐7. [MEDLINE: ] - PubMed
-
- Fine RN, Kohaut EC, Brown D, Perlman AJ. Growth after recombinant human growth hormone treatment in children with chronic renal failure: report of a multicenter randomized double‐blind placebo‐controlled study. Genentech Cooperative Study Group. Journal of Pediatrics 1994;124(3):374‐82. [MEDLINE: ] - PubMed
Fine 2002 (TP) {published data only}
-
- Fine RN, Stablein D, Cohen AH, Tejani A, Kohaut E. Recombinant human growth hormone post‐renal transplantation in children: a randomized controlled study of the NAPRTCS. Kidney International 2002;62(2):688‐96. [MEDLINE: ] - PubMed
Hertel 2002 (preD/Dial) {published data only}
-
- Hertel NT, Holmberg C, Rönnholm KAR, Jacobsen BB, Ølgaard, Meeuwisse GW, et al. Recombinant human growth hormone treatment, using two dose regimens in children with chronic renal failure‐‐a report on linear growth and adverse effects. Journal of Pediatric Endocrinology 2002;15(5):577‐88. [MEDLINE: ] - PubMed
Hokken‐K 1991 (preD/Dial) {published data only}
-
- Hokken‐Koelega A, Mulder P, Jong R, Lilien M, Donckerwolcke R, Groothof J. Long‐term effects of growth hormone treatment on growth and puberty in patients with chronic renal insufficiency. Pediatric Nephrology 2000;14(7):701‐6. [MEDLINE: ] - PubMed
-
- Hokken‐Koelega AC, Stijnen T, Muinck Keizer‐Schrama SM, Wit JM, Wolff ED, Jong MC, et al. Placebo‐controlled, double‐blind, cross‐over trial of growth hormone treatment in prepubertal children with chronic renal failure. Lancet 1991;338(8767):585‐90. [MEDLINE: ] - PubMed
-
- Graaff LC, Mulder PG, Hokken‐Koelega AC. Body proportions before and during growth hormone therapy in children with chronic renal failure. Pediatric Nephrology 2003;18(7):679‐84. [MEDLINE: ] - PubMed
Hokken‐K 1994 (preD/Dial) {published data only}
-
- Hokken‐Koelega A, Mulder P, Jong R, Lilien M, Donckerwolcke R, Groothof J. Long‐term effects of growth hormone treatment on growth and puberty in patients with chronic renal insufficiency. Pediatric Nephrology 2000;14(7):701‐6. [MEDLINE: ] - PubMed
-
- Hokken‐Koelega AC, Stijnen T, Jong MC, Donckerwolcke RA, Muinck Keizer‐Schrama SM, Blum WF, et al. Double blind trial comparing the effects of two doses of growth hormone in prepubertal patients with chronic renal insufficiency. Journal of Clinical Endocrinology & Metabolism 1994;79(4):1185‐90. [MEDLINE: ] - PubMed
-
- Graaff LC, Mulder PG, Hokken‐Koelega AC. Body proportions before and during growth hormone therapy in children with chronic renal failure. Pediatric Nephrology 2003;18(7):679‐84. [MEDLINE: ] - PubMed
Hokken‐K 1994 (TP) {published data only}
-
- Hokken‐Koelega AC, Stijnen T, Ridder MA, Muinck Keizer‐Schrama SM, Wolff ED, Jong MC, et al. Growth hormone treatment in growth‐retarded adolescents after renal transplant. Lancet 1994;343(8909):1313‐7. [MEDLINE: ] - PubMed
Hokken‐K 1996 (TP) {published data only}
-
- Hokken‐Koelega AC, Sitjnen T, Jong RC, Donckerwolcke RA, Groothoff JW, Wolff ED, et al. A placebo‐controlled, double‐blind trial of growth hormone treatment in prepubertal children after renal transplant. Kidney International ‐ Supplement 1996;53:128‐34. [MEDLINE: ] - PubMed
Ito 1997 (TP) {published data only}
-
- Ito K, Kawaguchi H. The use of recombinant human GH (rhGH) in growth failure in children with various renal diseases. Clinical Pediatric Endocrinology 1997;6 Suppl(9):49‐53. [EMBASE: 1998192189]
-
- Ito K, Kawaguchi H and Multicenter Study Group of GH in Japan. A wide applicability of growth hormone (Genotrophin) to growth impairment in children suffering from various renal diseases [abstract]. Pediatric Nephrology 1995;9(6):C70.
-
- Ito K, Kawaguchi H, Shizume K, Hibi I. The effects of recombinant human growth hormone (r‐hGH, SM‐9500, genotropin) on growth disturbance in children after renal transplantation. Japanese Multi‐Center Study (Genotropin) Group on Children with Renal Disease. Nippon Jinzo Gakkai Shi. Japanese Journal of Nephrology 1995;37(3):194‐200. [MEDLINE: ] - PubMed
-
- Kawaguchi H, Ito K. rhGH use in children with CRI and undergoing dialysis post‐transplant in Japan: A multicentre study. British Journal of Clinical Practice Supplement 1996;85(Supplement 1):26‐31. - PubMed
Kitagawa 1997 (Dial) {published data only}
-
- Ito K, Kawaguchi H. The use of recombinant human GH (rhGH) in growth failure in children with various renal diseases. Clinical Pediatric Endocrinology 1997;6 Suppl(9):49‐53. [EMBASE: 1998192189]
-
- Ito K, Kawaguchi H and Japanese Multicenter Study Group. A dose modifying effects of growth hormone (GH:NN‐798, Norditrophin) in children with chronic renal insufficiency (CRI) [abstract]. Pediatric Nephrology 1995;9(6):C71.
-
- Kawaguchi H, Ito K. rhGH use in children with CRI and undergoing dialysis post‐transplant in Japan: A multicentre study. British Journal of Clinical Practice. Supplement 1996;85:26‐31. [MEDLINE: ] - PubMed
-
- Kitagawa T, Ito K, Ito H, Sakai T, Wada H, Kajiwara N. GH treatment of children with chronic renal insufficiency: A Japanese clinical trial. Clinical Pediatric Endocrinology 1997;6 Suppl(10):73‐80. [MEDLINE: ]
Kitagawa 1997 (preD) {published data only}
-
- Ito K, Kawaguchi H. The use of recombinant human GH (rhGH) in growth failure in children with various renal diseases. Clinical Pediatric Endocrinology 1997;6 Suppl(9):49‐53. [EMBASE: 1998192189]
-
- Ito K, Kawaguchi H and Japanese Multicenter Study Group. A dose modifying effects of growth hormone (GH:NN‐798, Norditrophin) in children with chronic renal insufficiency (CRI) [abstract]. Pediatric Nephrology 1995;9(6):C71.
-
- Kawaguchi H, Ito K. rhGH use in children with CRI and undergoing dialysis post‐transplant in Japan: A multicentre study. British Journal of Clinical Practice. Supplement 1996;85:26‐31. [MEDLINE: ] - PubMed
-
- Kitagawa T, Ito K, Ito H, Sakai T, Wada H, Kajiwara N. GH treatment of children with chronic renal insufficiency: A Japanese clinical trial. Clinical Pediatric Endocrinology 1997;6 Suppl(10):73‐80. [EMBASE: 1998182276]
Kuizon 1998 (Dial) {published data only}
-
- Kuizon BD, Goodman WG, Gales B, Juppner H, Salusky IB. Effects of growth hormone on bone and mineral metabolism in dialyzed children [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):546‐7.
-
- Kuizon BD, Goodman WG, Gales B, Sahney S, Juppner H, Salusky IB. Growth hormone therapy inhibits the suppressive effects of calcitriol on bone in patients with secondary hyperparathyroidism [abstract]. Pediatric Nephrology 2001;16:C48.
Maxwell 1998a (TP) {published data only}
Maxwell 1998b (TP) {published data only}
Powell 1997 (preD) {published data only}
-
- Brewer ED, Ou C, Hogg RJ. Glomerular filtration rate (GFR) in children with advanced chronic renal failure (CRF) during short term therapy with growth hormone (rhGH)‐‐ a multicenter trial of the southwest pediatric nephrology study group (SPNSG) [abstract]. Journal of the American Society of Nephrology 1994;5(3):326.
-
- Powell DR, Attie K, Hintz RL, Liu F, Baker B, Kuntze J, et al. Growth hormone stimulates growth and alters the serum insulin‐growth factor axis of children with chronic renal failure: Report of the Southwest Pediatric Nephrology Study Group [abstract]. Pediatric Nephrology 1995;9(6):C70.
-
- Powell DR, Durham SK, Brewer ED, Frane JW, Watkins SL, Hogg RJ, et al. Effects of chronic renal failure and growth hormone on serum levels of insulin‐like growth factor binding protein‐4 (IGFBP‐4) and IGFBP‐5 in children: a report of the Southwest Pediatric Nephrology Study Group. Journal of Clinical Endocrinology & Metabolism 1999;84(2):596‐601. [MEDLINE: ] - PubMed
-
- Powell DR, Liu F, Baker BK, Hintz RL, Lee PD, Durham SK, et al. Modulation of growth factors by growth hormone in children with chronic renal failure. The Southwest Pediatric Nephrology Study Group. Kidney International 1997;51(6):1970‐9. [MEDLINE: ] - PubMed
-
- Watkins SL, Hogg RJ, Brewer ED, Powell DR, Boechat I, Brown D, et al. Bone mineral parameters in children with chronic renal fialure (CRF) receiving recombinant human growth hormone (GH). A report of the Southwest Pediatric Nephrology Group. [abstract]. Pediatric Nephrology 1995;9(6):C51.
Sanchez 2002 (TP) {published data only}
-
- Sanchez CP, Goodman WG, Kuison BD, Gales B, Malekzadeh M, Saluusky IB. Growth hormone and bone: A randomised prospective study. 16th Annual Meeting. American Society of Transplant Physicians; 1997 May 10‐14; Chicago (USA). 1997:156.
-
- Sanchez CP, Goodman WG, Kuizon BD, Ettenger RB, Salusky IB. Growth response to growth hormone therapy varies with bone histology in pediatric renal transplant recipients [abstract]. Journal of the American Society of Nephrology 1998;9(Progams & Abstracts):550.
-
- Sanchez CP, Kuizon BD, Goodman WG, Gales B, Ettenger RB, Inez Boechat M, et al. Growth hormone and the skeleton in pediatric renal allograft recipients. Pediatric Nephrology 2002;17(5):322‐8. [MEDLINE: ] - PubMed
-
- Sanchez CP, Salem M, Ettenger RB. Changes in cyclosporine A levels in pediatric renal allograft recipients receiving recombinant human growth hormone therapy. Transplantation Proceedings 2000;32(8):2807‐10. [MEDLINE: ] - PubMed
Santos 2010 (preD/Dial) {published data only}
-
- Moreno L, Santos F, Neto A, Ariceta G, Vara J, Alonso A, et al. Improvement in growth after one year of growth hormone therapy in well‐nourished infants with growth retardation secondary to chronic renal failure: results of a multi‐center, controlled, randomized, open clinical trial [abstract]. Pediatric Nephrology 2010;25:790. - PMC - PubMed
-
- Santos F, Moreno ML, Neto A, Ariceta G, Vara J, Alonso A, et al. Improvement in growth after 1 year of growth hormone therapy in well‐nourished infants with growth retardation secondary to chronic renal failure: Results of a multicenter, controlled, randomized, open clinical trial. Clinical Journal of the American Society of Nephrology 2010;5(7):1190‐7. [MEDLINE: ] - PMC - PubMed
References to studies excluded from this review
Ghio 2002 (TP) {published data only}
-
- Ghio L, Damiani B, Garavaglia R, Oppizzi G, Taioli E, Edefonti A. Lipid profile during rhGH therapy in pediatric renal transplant patients. Pediatric Transplantation 2002;6(2):127‐31. [MEDLINE: ] - PubMed
Jedrzejowski 2002 {published data only}
-
- Jedrzejowski A, Panczyk‐Tomaszewska M, Roszkowska‐Blaim M, Lis D, Galazka B, Dyras P. Growth hormone therapy and lipid profile in children on chronic peritoneal dialysis. Pediatric Nephrology 2002;17(10):830‐6. [MEDLINE: ] - PubMed
Laine 1995 {published data only}
-
- Laine J, Krogerus L, Sipila I, Ronnholm K, Holmberg C. Effects of recombinant human growth hormone treatment of renal allograft function and histopathology [abstract]. Pediatric Nephrology 1995;9(6):49.
Additional references
Berard 2008
-
- Berard E, Andre JL, Guest G, Berthier F, Afanetti M, Cochat P, et al. Long‐term results of rhGH treatment in children with renal failure: experience of the French Society of Pediatric Nephrology. Pediatric Nephrology 2008;23(11):2031‐8. [MEDLINE: ] - PubMed
Clayton 2000
-
- Clayton PE, Cowell CT. Safety issues in children and adolescents during Growth Hormone Therapy ‐ a review. Growth Hormone & IgF Research 2000;10(6):306‐17. [MEDLINE: ] - PubMed
Cowell 1995
-
- Cowell CT, Dietsch S. Adverse events during growth hormone therapy. Journal of Pediatric Endocrinology & Metabolism 1995;8(4):243‐52. [MEDLINE: ] - PubMed
Dickersin 1995
Disney 1999
-
- Disney APS, Russ GR, Walker R, Sheil AGR, Collins J, Herbert K, Kerr P. ANZDATA Registry Report 1999. Adelaide: Australia and New Zealand Dialysis and Transplant Registry, 1999.
Fine 1995a
-
- Fine RN. Recombinant human growth hormone (rhGH) in dialysis patient: update 1995. Advances in Peritoneal Dialysis 1995;11:261‐5. [MEDLINE: ] - PubMed
Fine 2005
-
- Fine RN, Stablein D. Long‐term use of recombinant human growth hormone in pediatric allograft recipients: a report of the NAPRTCS Transplant Registry. Pediatric Nephrology 2005;20(3):404‐8. [MEDLINE: ] - PubMed
Fine 2010
-
- Fine RN, Martz K, Stablein D. What have 20 years of data from the North American Pediatric Renal Transplant Cooperative Study taught us about growth following renal transplantation in infants, children and adolescents with end‐stage renal disease?. Pediatric Nephrology 2010;25(4):739‐46. [MEDLINE: ] - PubMed
Haffner 2000
-
- Haffner D, Schaefer F, Nissel R, Wuhl E, Tönshoff B, Mehls O. Effect of growth hormone treatment on the adult height of children with chronic renal failure. New England Journal of Medicine 2000;343(13):923‐30. [MEDLINE: ] - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
K/DOQI 2002
-
- National Kidney Foundation. K/DOQI Workgroup. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. American Journal of Kidney Diseases 2002;39(2 Suppl 1):1‐266. [MEDLINE: ] - PubMed
Lefebvre 1996
-
- Lefebvre D, McDonald S. Development of a sensitive search strategy for reports of randomised controlled trials in Embase. Fourth International Cochrane Colloquium; 1996 Oct 20‐24; Adelaide (Australia). 1996:20‐4.
Moyer 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐613. [MEDLINE: ] - PubMed
Pettiti 1994
-
- Pettiti DB. Meta‐analysis decision analysis and cost‐effectiveness analysis: methods for quantitative synthesis in medicine. New York: Oxford University Press, 1994.
Postlethwaite 1998
Reeves 2002
-
- Reeves GD, Doyle DA. Growth hormone treatment and pseudotumor cerebri: coincidence or close relationship?. Journal of Pediatric Endocrinology 2002;15 Suppl 2:723‐30. [MEDLINE: ] - PubMed
Reynolds 1995
Rizzoni 1985
-
- Rizzoni G, Broyer M, Brunner FP, Brynger H, Challah S, Kramer P, et al. Combined report on regular dialysis and transplantation of children in Europe, XIII, 1983. Proceedings of the European Dialysis & Transplant Association ‐ European Renal Association. 1985; Vol. 21:66‐95. [MEDLINE: ] - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] - PubMed
Tönshoff 1995
-
- Tönshoff B, Mehls O. Growth retardation in children with chronic renal insufficiency: current aspects of pathophysiology and treatment. Journal of Nephrology 1995;8(3):133‐42. [EMBASE: 1995200628]
Wilton 1999
-
- Wilton P. Adverse events during GH treatment: 10 years' experience in KIGS, a pharmacoepidemiological survey. In: Ranke MB, Wilton P editor(s). Progress in growth hormone therapy ‐ 10 years of KIGS. Mannheim: Ja Barh Verlag, 1999:349‐64.
Wühl 1996
-
- Wühl E, Haffner N, Nissel R, Schaefer F, Mehls O and German Study Group for Growth Hormone Treatment in Chronic Renal Failure. Short dialyzed children respond less to growth hormone than patients prior to dialysis. Pediatric Nephrology 1996;10(3):294‐8. [MEDLINE: ] - PubMed
References to other published versions of this review
Vimalachandra 2001a
-
- Vimalachandra D, Craig JC, Cowell C, Knight JF. Growth hormone treatment in children with chronic renal failure: a meta‐analysis of randomized controlled trials. Journal of Pediatrics 2001;139(4):560‐6. [MEDLINE: ] - PubMed
Vimalachandra 2001b
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

