Antibiotics for leptospirosis
- PMID: 22336839
- PMCID: PMC11299142
- DOI: 10.1002/14651858.CD008264.pub2
Antibiotics for leptospirosis
Abstract
Background: Leptospirosis has a wide-ranging clinical and public health impact. Leptospira are globally distributed. Case attack rates are as high as 1:4 to 2:5 persons in exposed populations. In some settings mortality has exceeded 10% of infected people. The benefit of antibiotic therapy in the disease has been unclear.
Objectives: We sought to characterise the risks and benefits associated with use of antibiotic therapy in the management of leptospirosis.
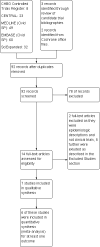
Search methods: We searched the The Cochrane Hepato-Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded regardless of study language. This was augmented by a manual search. The last date of search was November, 2011.
Selection criteria: To be included in assessment of benefits, trials had to specifically assess the use of antibiotics in a randomised clinical trial. A broad range of study types were incorporated to seek potential harms.
Data collection and analysis: Included trials were systematically abstracted, as were excluded studies for the purposes of assessing harms. Analyses were conducted in accordance with The Cochrane Handbook and practices of The Cochrane Hepato-Biliary Group.
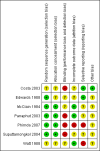
Main results: Seven randomised trials were included. Four trials with 403 patients compared an antibiotic with placebo or no intervention. Three trials compared at least one antibiotic regimen with another antibiotic regimen. The trials all had high risk of bias. The trials varied in the severity of leptospirosis among trial patients. The ability to group data for meta-analysis was limited. While all four trials that compared antibiotics with placebo reported mortality and used parenteral penicillin, there were no deaths in two of them. Since odds ratio calculations cannot employ zero-event trials, only two trials contributed to this estimate. The number of deaths were 16/200 (8.0%) in the antibiotic arm versus 11/203 (5.4%) in the placebo arm giving a fixed-effect OR 1.56 (95% CI 0.70 to 3.46). The random-effects OR is 1.16 (95% CI 0.23 to 5.95). The heterogeneity among these four trials for the mortality outcome was moderate (I(2)= 50%). Only one trial (253 patients) reported days of hospitalisation. It compared parenteral penicillin to placebo without significant effect of therapy (8.9 versus 8.8 days; mean difference (MD) 0.10 days, 95% CI -0.83 to 1.03). The difference in days of clinical illness was reported in two of these trials (71 patients). While parenteral penicillin therapy conferred 4.7 to 5.6 days of clinical illness in contrast to 7.7 to 11.6 days in the placebo arm, the size of the estimate of effect increased but statistical significance was lost under the random-effect model (fixed-effect: MD -2.13 days, 95% CI -2.46 to -1.80; random-effects: MD -4.04, 95% CI -8.66 to 0.58). I(2) for this outcome was high (81%). When duration of fever alone was assessed between antibiotics and placebo in a single trial (79 patients), no significant difference existed (6.9 versus 6.6 days; MD 0.30, 95% CI -1.26 to 1.86). Two trials with 332 patients in relatively severe and possibly late leptospirosis, resulted in trends towards increased dialysis when penicillin was used rather than placebo, but the estimate of effect was small and did not reach statistical significance (42/163 (25.8%) versus 31/169 (18.4%); OR 1.54, 95% CI 0.91 to 2.60). When one antibiotic was assessed against another antibiotic, there were no statistically significant results. For mortality in particular, these comparisons included cephalosporin versus penicillin (2 trials, 6/176 (3.4%) versus 9/175 (5.2%); fixed-effect: OR 0.65, 95% CI 0.23 to 1.87, I(2)= 16%), doxycycline versus penicillin (1 trial, 2/81 (2.5%) versus 4/89 (4.5); OR 0.54, 95% CI 0.10 to 3.02), cephalosporin versus doxycycline (1 trial, 1/88 (1.1%) versus 2/81 (2.5%); OR 0.45, 95% CI 0.04 to 5.10). There were no adverse events of therapy which reached statistical significance.
Authors' conclusions: Insufficient evidence is available to advocate for or against the use of antibiotics in the therapy for leptospirosis. Among survivors who were hospitalised for leptospirosis, use of antibiotics for leptospirosis may have decreased the duration of clinical illness by two to four days, though this result was not statistically significant. When electing to treat with an antibiotic, selection of penicillin, doxycycline, or cephalosporin does not seem to impact mortality nor duration of fever. The benefit of antibiotic therapy in the treatment of leptospirosis remains unclear, particularly for severe disease. Further clinical research is needed to include broader panels of therapy tested against placebo.
Conflict of interest statement
None known.
Figures


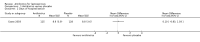


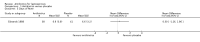

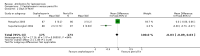




Update of
References
References to studies included in this review
Costa 2003 {published data only (unpublished sought but not used)}
-
- Costa E, Lopes AA, Sacramento E, Costa YA, Matos ED, Baretto M, et al. Penicillin at the late stage of leptospirosis: a randomized controlled trial. Revista do Instituto de Medicina Tropical de Sao Paulo 2003;45(3):141‐5. - PubMed
Edwards 1988 {published data only}
-
- Edwards CN, Nicholson GD, Hassell TA, Everard CO, Callender J. Penicillin therapy in icteric leptospirosis. American Journal of Tropical Medicine and Hygiene 1988;39(4):388‐90. - PubMed
McClain 1984 {published data only}
-
- McClain BL, Ballou WR, Harrison SM, Steinweg DL. Doxcycyline therapy for leptospirosis.. Annals of Internal Medicine 1984;100(5):696‐8. - PubMed
Panaphut 2003 {published data only (unpublished sought but not used)}
-
- Panaphut T, Domrongkitchaiporn S, Vibhagool A, Thinkamrop B, Susaengrat W. Ceftriaxone compared with sodium penicillin for treatment of severe leptospirosis. Clinical Infectious Diseases 2003;36:1507‐13. - PubMed
Phimda 2007 {published and unpublished data}
Suputtamongkol 2004 {published and unpublished data}
-
- Suputtamongkol Y, Niwattayakul K, Suttinont C, Losuwanaluk K, Limpaiboon R, Chierakul W, et al. An open, randomized, controlled trial of penicillin, doxycycline, and cefotaxime for patients with severe leptospirosis. Clinical Infectious Diseases 2004;39:1417‐24. - PubMed
Watt 1988 {published data only}
-
- Watt G, Padre LP, Tuazon ML, Calubaquib C, Santiago E, Ranoa CP, et al. Placebo‐controlled trial of intravenous penicillin for severe and late leptospirosis. Lancet 1988;1(8583):433‐5. - PubMed
References to studies excluded from this review
Doherty 1955 {published data only}
-
- Doherty RL. A clinical study of leptospirosis in North Queensland. Australas Annals of Medicine 1955;4(1):53‐63. - PubMed
Fairburn 1956 {published data only}
-
- Fairburn AC, Semple SJG. Chloramphenicol and penicillin in the treatment of leptospirosis among British troops in Malaya. Lancet 1956;270(6906):13‐6. - PubMed
Hall 1951 {published data only}
-
- Hall HE, Hightower JA, Diaz Rivera R, Byrne RJ, Smadel JE, Woodward TE. Evaluation of antibiotic therapy in human leptospirosis. Annals of Internal Medicine 1951;35(5):981‐98. - PubMed
Munnich 1972 {published data only}
-
- Munnich D, Lakatos M. Treatment of leptospirosis with Semicillin. Therapia Hungarica (English Edition) 1972;20(4):152‐5. - PubMed
Russell 1958 {published data only}
-
- Russell RW. Treatment of leptospirosis with oxytetracycline. Lancet 1958;2(7057):1143‐5. - PubMed
Additional references
Bharti 2003
-
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. The Lancet Infectious Diseases 2003;3(12):757‐71. - PubMed
Brett‐Major 2009
Egger 1997
Gluud 2011
-
- Gluud C, Nikolova D, Klingenberg SL, Whitfield K, Alexakis N, Als‐Nielsen B, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 8. Art. No.: LIVER.
Hadad 2006
-
- Hadad E, Pirogovsky A, Bartal C, Gilad J, Barnea A, Yitzhaki S, et al. An outbreak of leptospirosis among Israeli troops near the Jordan River. The American Journal of Tropical Medicine and Hygiene 2006;74(1):127‐31. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
ICH‐E3 1995
-
- ICH harmonized tripartite guideline. Guideline on structure and content of clinical study reports (E3). Geneva: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Eff... 1995 (accessed 16 May 2011).
ICH‐GCP 1996
-
- ICH harmonised tripartite guideline. Guideline for good clinical practice E6 (R1). Geneva: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Eff... 1996 (accessed 16 May 2011).
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. - PubMed
LaRocque 2005
Leandro 2005
-
- Leangro G. Meta‐analysis in Medical Research: The Handbook for the Understanding and Practice of Meta‐Analysis. Oxford: Blackwell Publishing Ltd., 2005.
Liverpool 2008
-
- Liverpool J, Francis S, Liverpool CE, Dean GT, Mendez DD. Leptospirosis: case reports of an outbreak in Guyana. Annals of Tropical Medicine and Parasitology 2008;102(3):239‐45. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Pappas 2008
-
- Pappas G, Papadimitriou P, Siozopoulo V, Christou L, Akritidis N. The globalization of leptospirosis: worldwide incidence trends. International Journal of Infectious Diseases 2008;12(4):351‐7. [MEDLINE: ] - PubMed
Ressner 2008
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Russell 2003
-
- Russell KL, Montiel Gonzalez MA, Watts DM, Lagos‐Figueroa RC, Chauca G, Ore M, et al. An outbreak of leptospirosis among Peruvian military recruits. The American Journal of Tropical Medicine and Hygiene 2003;69(1):53‐7. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. - PubMed
Sejvar 2003
WHO 2003
-
- World Health Organization. International Leptospirsos Society. Human Leptospirosis: Guide for Diagnosis, Surveillance and Control. World Health Organization, 2003.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

