Sumatriptan (oral route of administration) for acute migraine attacks in adults
- PMID: 22336849
- PMCID: PMC4167868
- DOI: 10.1002/14651858.CD008615.pub2
Sumatriptan (oral route of administration) for acute migraine attacks in adults
Abstract
Background: Migraine is a highly disabling condition for the individual and also has wide-reaching implications for society, healthcare services, and the economy. Sumatriptan is an abortive medication for migraine attacks, belonging to the triptan family.
Objectives: To determine the efficacy and tolerability of oral sumatriptan compared to placebo and other active interventions in the treatment of acute migraine attacks in adults.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, online databases, and reference lists for studies through 13 October 2011.
Selection criteria: We included randomised, double-blind, placebo- and/or active-controlled studies using oral sumatriptan to treat a migraine headache episode, with at least 10 participants per treatment arm.
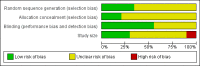
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. We used numbers of participants achieving each outcome to calculate relative risk (or 'risk ratio') and numbers needed to treat to benefit (NNT) or harm (NNH) compared to placebo or a different active treatment.
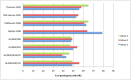
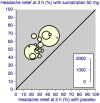
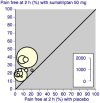
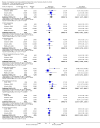
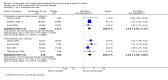
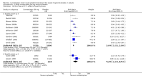
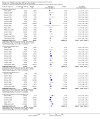
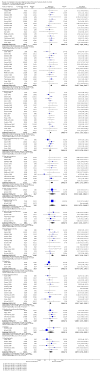
Main results: Sixty-one studies (37,250 participants) compared oral sumatriptan with placebo or an active comparator. Most of the data were for the 50 mg and 100 mg doses. Sumatriptan surpassed placebo for all efficacy outcomes. For sumatriptan 50 mg versus placebo the NNTs were 6.1, 7.5, and 4.0 for pain-free at two hours and headache relief at one and two hours, respectively. NNTs for sustained pain-free and sustained headache relief during the 24 hours postdose were 9.5 and 6.0, respectively. For sumatriptan 100 mg versus placebo the NNTs were 4.7, 6.8, 3.5, 6.5, and 5.2, respectively, for the same outcomes. Results for the 25 mg dose were similar to the 50 mg dose, while sumatriptan 100 mg was significantly better than 50 mg for pain-free and headache relief at two hours, and for sustained pain-free during 24 hours. Treating early, during the mild pain phase, gave significantly better NNTs for pain-free at two hours and sustained pain-free during 24 hours than did treating established attacks with moderate or severe pain intensity.Relief of associated symptoms, including nausea, photophobia, and phonophobia, was greater with sumatriptan than with placebo, and use of rescue medication was lower with sumatriptan than with placebo. For the most part, adverse events were transient and mild and were more common with the sumatriptan than with placebo, with a clear dose response relationship (25 mg to 100 mg).Sumatriptan was compared directly with a number of active treatments, including other triptans, paracetamol (acetaminophen), acetylsalicylic acid, non-steroidal anti-inflammatory drugs (NSAIDs), and ergotamine combinations.
Authors' conclusions: Oral sumatriptan is effective as an abortive treatment for migraine attacks, relieving pain, nausea, photophobia, phonophobia, and functional disability, but is associated with increased adverse events.
Conflict of interest statement
RAM and SD have received research support from charities, government, and industry sources at various times. RAM has consulted for various pharmaceutical companies, including GlaxoSmithKline, the manufacturers of sumatriptan. RAM has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. CD has no interests to declare. GlaxoSmithKline were not in any way involved in conducting this review.
Figures






















































































































Update of
-
WITHDRAWN: Oral sumatriptan for acute migraine.Cochrane Database Syst Rev. 2012 Feb 15;2012(2):CD002915. doi: 10.1002/14651858.CD002915.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2012 Feb 15;(2):CD008615. doi: 10.1002/14651858.CD008615.pub2. PMID: 22336784 Free PMC article. Updated.
References
References to studies included in this review
160‐104 {unpublished data only}
-
- A multicentre, double‐blind, placebo controlled, parallel group study of two dose levels of oral eletriptan and two dose levels of oral sumatriptan given for the acute treatment of migraine. Pfizer clinical trial report 1998.
Banerjee 1992 {published data only}
-
- Banerjee M, Findley LJ. Sumatriptan in the treatment of acute migraine with aura. Cephalalgia 1992;12(1):39‐44. - PubMed
Brandes 2007 {published data only}
-
- Brandes JL, Kudrow D, Stark SR, O'Carroll CP, Adelman JU, O'Donnell FJ, et al. Sumatriptan‐naproxen for acute treatment of migraine: a randomized trial. JAMA 2007;297(13):1443‐54. - PubMed
-
- Landy S, DeRossett SE, Rapoport A, Rothrock J, Ames MH, McDonald SA, et al. Two double‐blind, multicenter, randomized, placebo‐controlled, single‐dose studies of sumatriptan/naproxen sodium in the acute treatment of migraine: function, productivity, and satisfaction outcomes. MedGenMed 2007;9(2):53. - PMC - PubMed
Bussone 2000 {published data only}
Carpay 2004 {published data only}
-
- Barbanti P, Carpay JA, Kwong WJ, Ahmad F, Boswell D. Effects of a fast disintegrating/rapid release oral formulation of sumatriptan on functional ability in patients with migraine. Current Medical Research and Opinion 2004;20(12):2021‐9. - PubMed
-
- Carpay J, Schoenen J, Ahmad F, Kinrade F, Boswell D. Efficacy and tolerability of sumatriptan tablets in a fast‐disintegrating, rapid‐release formulation for the acute treatment of migraine: results of a multicenter, randomized, placebo‐controlled study. Clinical Therapeutics 2004;26(2):214‐23. [DOI: ] - PubMed
Cutler 1995 {published data only}
-
- Cutler N, Mushet GR, Davis R, Clements B, Whitcher L. Oral sumatriptan for the acute treatment of migraine: evaluation of three dosage strengths. Neurology 1995;45(8 Suppl 7):S5‐9. - PubMed
Dahlof 1991 {published data only}
-
- The Oral Sumatriptan Dose‐Defining Study Group. Sumatriptan‐‐an oral dose‐defining study. European Neurology 1991;31(5):300‐5. - PubMed
Dahlof 2009 {published data only}
-
- Dahlof CG, Hauge AW, Olesen J. Efficacy and safety of tonabersat, a gap‐junction modulator, in the acute treatment of migraine: a double‐blind, parallel‐group, randomized study. Cephalalgia 2009;29 Suppl 2:7‐16. [PUBMED: 19723121] - PubMed
Diener 2004a {published data only}
Diener 2004b {published data only}
-
- Diener HC, Bussone G, Liano H, Eikermann A, Englert R, Floeter T, et al. Placebo‐controlled comparison of effervescent acetylsalicylic acid, sumatriptan and ibuprofen in the treatment of migraine attacks. Cephalalgia 2004;24(11):947‐54. [PUBMED: 15482357] - PubMed
DKSMSG 1999 {published data only}
-
- The Diclofenac‐K/Sumatriptan Migraine Study Group. Acute treatment of migraine attacks: efficacy and safety of a nonsteroidal anti‐inflammatory drug, diclofenac‐potassium, in comparison to oral sumatriptan and placebo. Cephalalgia 1999;19(4):232‐40. - PubMed
Dodick 2002 {published data only}
Dowson 2002 {published data only}
-
- Dowson AJ, Massiou H, Lainez JM, Cabarrocas X. Almotriptan improves response rates when treatment is within 1 hour of migraine onset. Headache 2004;44(4):318‐22. - PubMed
-
- Dowson AJ, Massiou H, LaÍnez JM, Cabarrocas X. Almotriptan is an effective and well‐tolerated treatment for migraine pain: results of a randomized, double‐blind, placebo‐controlled clinical trial. Cephalalgia 2002;22(6):453‐61. [PUBMED: 12133045] - PubMed
Ensink 1991 {published data only}
-
- Ensink FB, Sumatriptan International Study Group. Subcutaneous sumatriptan in the acute treatment of migraine. Journal of Neurology 1991;238 Suppl 1:S66‐9. [PUBMED: 1646291] - PubMed
Freitag 2001 {published data only}
-
- Freitag FG, Cady R, DiSerio F, Elkind A, Gallagher RM, Goldstein J, et al. Comparative study of a combination of isometheptene mucate, dichloralphenazone with acetaminophen and sumatriptan succinate in the treatment of migraine. Headache 2001;41(4):391‐8. [DOI: 10.1046/j.1526-4610.2001.111006391.x] - DOI - PubMed
Gallagher 2000 {published data only}
Geraud 2000 {published data only}
-
- Geraud G, Olesen J, Pfaffenrath V, Tfelt‐Hansen P, Zupping R, Diener HC, et al. Comparison of the efficacy of zolmitriptan and sumatriptan: issues in migraine trial design. Cephalalgia 2000;20(1):30‐8. [PUBMED: 10817444] - PubMed
GL/MIG/001/92 {unpublished data only}
-
- A double‐blind, general practice study to compare GR43175 with paracetamol and metoclopramide in the acute treatment of migraine (Original protocol). www.gsk‐clinicalstudyregister.com/ 1992.
GL/MIG/001A/92 {unpublished data only}
-
- A double‐blind, general practice study to compare GR43175 with paracetamol and metoclopramide in the acute treatment of migraine (Amended protocol). www.gsk‐clinicalstudyregister.com/ 1992.
GL/MIG/002 {unpublished data only}
-
- A double‐blind general practice study to compare sumatriptan with Migraleve in the acute treatment of migraine (Original protocol). www.gsk‐clinicalstudyregister.com/ 1990.
GL/MIG/002A {unpublished data only}
-
- A double‐blind general practice study to compare sumatriptan with Migraleve in the acute treatment of migraine (Amended protocol). www.gsk‐clinicalstudyregister.com/ 1991.
GL/MIG/009 {unpublished data only}
-
- A double blind study to compare acute sumatriptan with Migril in the treatment of migraine. www.gsk‐clinicalstudyregister.com/ 1991.
Goadsby 1991 {published data only}
Goadsby 2000 {published data only}
-
- Goadsby PJ, Ferrari MD, Olesen J, Stovner LJ, Senard JM, Jackson NC, et al. Eletriptan Steering Committee. Eletriptan in acute migraine: a double‐blind, placebo‐controlled comparison to sumatriptan. Neurology 2000;54(1):156‐63. - PubMed
Goldstein 1998 {published data only}
Goldstein 2005 {published data only}
Gruffyd‐Jones 2001 {published data only}
-
- Gruffyd‐Jones K, Kies B, Middleton A, Mulder LJ, Røsjø Ø, Millson DS. Zolmitriptan versus sumatriptan for the acute oral treatment of migraine: a randomized, double‐blind, international study. European Journal of Neurology 2001;8(3):237‐45. - PubMed
Havanka 2000 {published data only}
Ishkanian 2007 {published data only}
-
- Ishkanian G, Blumenthal H, Webster CJ, Richardson MS, Ames M. Efficacy of sumatriptan tablets in migraineurs self‐described or physician‐diagnosed as having sinus headache: a randomized, double‐blind, placebo‐controlled study. Clinical Therapeutics 2007;29(1):99‐109. [DOI: 10.1016/j.clinthera.2007.01.012] - DOI - PubMed
Jelinski 2006 {published data only}
-
- Jelinski SE, Becker WJ, Christie SN, Ahmad FE, Pryse‐Phillips W, Simpson SD. Pain free efficacy of sumatriptan in the early treatment of migraine. Canadian Journal of Neurological Sciences 2006;33(1):73‐9. [http://cjns.metapress.com/openurl.asp?genre=article&issn=0317‐1671&a... - PubMed
-
- SUM40291. A randomized, double‐blind, placebo‐controlled, parallel‐group, single‐attack evaluation of sumatriptan 50 mg and 100 mg versus placebo during a migraine headache at the first sign of pain. www.gsk‐clinicalstudyregister.com/ 2001.
Kaniecki 2006 {published data only}
Kolodny 2004 {published data only}
-
- Kolodny A, Polis A, Battisti WP, Johnson‐Pratt L, Skobieranda F, Rizatriptan Protocol 052 Study Group. Comparison of rizatriptan 5 mg and 10 mg tablets and sumatriptan 25 mg and 50 mg tablets. Cephalalgia 2004;24(7):540‐6. - PubMed
Kudrow 2005 {published data only}
Latere 1991 {published data only}
-
- The Multinational Oral Sumatriptan and Cafergot Comparative Study Group. A randomized, double‐blind comparison of sumatriptan and Cafergot in the acute treatment of migraine. European Neurology 1991;31(5):314‐22. - PubMed
Lines 2001 {published data only}
Lipton 2000 {published data only}
Mathew 2003 {published data only}
Myllyla 1998 {published data only}
-
- Myllylä VV, Havanka H, Herrala L, Kangasniemi P, Rautakorpi I, Turkka J, et al. Tolfenamic acid rapid release versus sumatriptan in the acute treatment of migraine: comparable effect in a double‐blind, randomized, controlled, parallel‐group study. Headache 1998;38(3):201‐7. [DOI: 10.1046/j.1526-4610.1998.3803201.x] - DOI - PubMed
Nappi 1994 {published data only}
-
- Nappi G, Sicuteri F, Byrne M, Roncolato M, Zerbini O. Oral sumatriptan compared with placebo in the acute treatment of migraine. Journal of Neurology 1994;241(3):138‐44. - PubMed
Nett 2003 {published data only}
-
- Nett R, Landy S, Shackelford S, Richardson MS, Ames M, Lener M. Pain‐free efficacy after treatment with sumatriptan in the mild pain phase of menstrually associated migraine. Journal of Obstetrics and Gynecology 2003;102(4):835‐42. - PubMed
Patten 1991 {published data only}
-
- Patten JP. Clinical experience with oral sumatriptan: a placebo‐controlled, dose‐ranging study. Oral Sumatriptan Dose‐defining Study Group. Journal of Neurology 1991;238 Suppl 1:S62‐5. - PubMed
Pfaffenrath 1998 {published data only}
Pini 1995 {published data only}
-
- Pini LA, Sternieri E, Fabbri L, Zerbini O, Bamfi F, The Oral Sumatriptan Italian Study Group. High efficacy and low frequency of headache recurrence after oral sumatriptan. Journal of International Medical Research 1995;23(2):96‐105. - PubMed
Pini 1999 {published data only}
-
- Pini LA, Fabbri L, Cavazzuti L, The Sumatriptan 50 mg Italian Study Group. Efficacy and safety of sumatriptan 50 mg in patients not responding to standard care, in the treatment of mild to moderate migraine. International Journal of Clinical Pharmacology Research 1999;19(2):57‐64. - PubMed
Sandrini 2002 {published data only}
-
- Sandrini G, Färkkilä M, Burgess G, Forster E, Haughie S, Eletriptan Steering Committee. Eletriptan vs sumatriptan: a double‐blind, placebo‐controlled, multiple migraine attack study. Neurology 2002;59(8):1210‐7. - PubMed
Sandrini 2007 {published data only}
-
- Sandrini G, Cerbo R, Bene E, Ferrari A, Genco S, Grazioli I, et al. Efficacy of dosing and re‐dosing of two oral fixed combinations of indomethacin, prochlorperazine and caffeine compared with oral sumatriptan in the acute treatment of multiple migraine attacks: a double‐blind, double‐dummy, randomised, parallel group, multicentre study. International Journal of Clinical Practice 2007;61(8):1256‐69. [DOI: 10.1111/j.1742-1241.2007.01458.x] - DOI - PMC - PubMed
Sargent 1995 {published data only}
-
- Sargent J, Kirchner JR, Davis R, Kirkhart B. Oral sumatriptan is effective and well tolerated for the acute treatment of migraine: results of a multicenter study. Neurology 1995;45(8 Suppl 7):S10‐4. - PubMed
Savani 1999 {published data only}
-
- Savani N, Brautaset NJ, Reunanen M, Szirmai I, Ashford EA, Hassani H, et al. Sumatriptan Tablets S2CM07 Study Group. A double‐blind placebo‐controlled study assessing the efficacy and tolerability of 50 mg sumatriptan tablets in the acute treatment of migraine. International Journal of Clinical Practice. Supplement 1999;105:7‐15. - PubMed
Schulman 2003 {published data only}
Sheftell 2005 {published data only}
-
- Sheftell FD, Dahlöf CG, Brandes JL, Agosti R, Jones MW, Barrett PS. Two replicate randomized, double‐blind, placebo‐controlled trials of the time to onset of pain relief in the acute treatment of migraine with a fast‐disintegrating/rapid‐release formulation of sumatriptan tablets. Clinical Therapeutics 2005;27(4):407‐17. - PubMed
Smith 2005 {published data only}
Spierings 2001 {published data only}
-
- Spierings EL, Gomez‐Mancilla B, Grosz DE, Rowland CR, Whaley FS, Jirgens KJ. Oral almotriptan vs. oral sumatriptan in the abortive treatment of migraine: a double‐blind, randomized, parallel‐group, optimum‐dose comparison. Archives of Neurology 2001;58(6):944‐50. - PubMed
Tfelt‐Hansen 1995 {published data only}
-
- Tfelt‐Hansen P, Henry P, Mulder LJ, Scheldewaert RG, Schoenen J, Chazot G. The combination of oral lysine‐acetylsalicylate and metoclopramide compared with oral sumatriptan in the treatment of migraine attacks. A randomized, double‐blind, placebo‐controlled clinical trial. Ugeskrift for Laeger 1996;158(45):6435‐9. - PubMed
-
- Tfelt‐Hansen P, Henry P, Mulder LJ, Scheldewaert RG, Schoenen J, Chazot G. The effectiveness of combined oral lysine acetylsalicylate and metoclopramide compared with oral sumatriptan for migraine. Lancet 1995;346(8980):923‐6. - PubMed
Tfelt‐Hansen 1998 {published data only}
Tfelt‐Hansen 2006 {published data only}
-
- Tfelt‐Hansen P, Bach FW, Daugaard D, Tsiropoulos I, Riddersholm B. Treatment with sumatriptan 50 mg in the mild phase of migraine attacks in patients with infrequent attacks: a randomised, double‐blind, placebo‐controlled study. Journal of Headache and Pain 2006;7(6):389‐94. [DOI: 10.1007/s10194-006-0333-z] - DOI - PMC - PubMed
Thomson 1992 {published data only}
-
- The Oral Sumatriptan and Aspirin plus Metoclopramide Comparative Study Group. A study to compare oral sumatriptan with oral aspirin plus oral metoclopramide in the acute treatment of migraine. European Neurology 1992;32(3):177‐84. - PubMed
Visser 1996 {published data only}
-
- Visser WH, Terwindt GM, Reines SA, Jiang K, Lines CR, Ferrari MD. Rizatriptan vs sumatriptan in the acute treatment of migraine. A placebo‐controlled, dose‐ranging study. Dutch/US Rizatriptan Study Group. Archives of Neurology 1996;53(11):1132‐7. - PubMed
Winner 2003 {published data only}
-
- Winner P, Mannix LK, Putnam DG, McNeal S, Kwong J, O'Quinn S, et al. Pain‐free results with sumatriptan taken at the first sign of migraine pain: 2 randomized, double‐blind, placebo‐controlled studies. Mayo Clinic Proceedings 2003;78(10):1214‐22. - PubMed
References to studies excluded from this review
Cady 1994 {published data only}
-
- Cady RK, Rubino J, Crummett D, Littlejohn TW 3rd. Oral sumatriptan in the treatment of recurrent headache. Archives of Family Medicine 1994;3(9):766‐72. - PubMed
Cady 2000 {published data only}
-
- Cady RK, Lipton RB, Hall C, Stewart WF, O'Quinn S, Gutterman D. Treatment of mild headache in disabled migraine sufferers: results of the Spectrum Study. Headache 2000;40(10):792‐7. - PubMed
Centonze 1995 {published data only}
-
- Centonze V, Polito MB, Bari M, Fabbri L, Cassiano MA, Bassi A, et al. Evaluation of the efficacy of oral sumatriptan in the management of migraine attacks. Clinical results [Italian]. La Clinica Terapeutica 1995;146(11):721‐8. - PubMed
Colman 2001 {published data only}
-
- Colman SS, Brod MI, Krishnamurthy A, Rowland CR, Jirgens KJ, Gomez‐Mancilla B. Treatment satisfaction, functional status, and health‐related quality of life of migraine patients treated with almotriptan or sumatriptan. Clinical Therapeutics 2001;23(1):127‐45. - PubMed
Dowson 2000 {published data only}
-
- Dowson A, Ball K, Haworth D. Comparison of a fixed combination of domperidone and paracetamol (Domperamol) with sumatriptan 50 mg in moderate to severe migraine: a randomised UK primary care study. Current Medical Research and Opinion 2000;16(3):190‐7. [DOI: ] - PubMed
Dowson 2005 {published data only}
Ferrari 1994 {published data only}
-
- Ferrari MD, James MH, Bates D, Pilgrim A, Ashford E, Anderson BA, et al. Oral sumatriptan: effect of a second dose, and incidence and treatment of headache recurrences. Cephalalgia 1994;14(5):330‐8. - PubMed
Gobel 2000 {published data only}
Landy 2004 (Study 1) {published data only}
Midelfart 1994 {published data only}
-
- Midelfart E, Winnem M. Sumatriptan in the treatment of migraine in general practice [Norwegian]. Tidsskr Nor Laegeforen 1994;114(29):3430‐2. - PubMed
Padma 1998 {published data only}
-
- Padma MV, Jain S, Maheshwari MC, Misra S, Karak B, Singh AK, et al. Efficacy and tolerability of oral sumatriptan in Indian patients with acute migraine; a multicentre study. Neurology India 1998;46(2):105‐8. - PubMed
Pradel 2005 {published data only}
-
- Pradel FG, Subedi P, Varghese AA, Mullins CD, Weis KA. Does earlier headache response equate to earlier return to functioning in patients suffering from migraine?. Cephalalgia 2005;26(4):428‐35. - PubMed
Rapoport 1995 {published data only}
-
- Rapoport AM, Visser WH, Cutler NR, Alderton CJ, Paulsgrove LA, Davis RL, et al. Oral sumatriptan in preventing headache recurrence after treatment of migraine attacks with subcutaneous sumatriptan. Neurology 1995;45(8):1505‐9. - PubMed
Rederich 1995 {published data only}
-
- Rederich G, Rapoport A, Cutler N, Hazelrigg R, Jamerson B. Sumatriptan for the long‐term treatment of migraine: clinical findings. Neurology 1995;45(8 Suppl 7):S15‐20. - PubMed
Salonen 1999 {published data only}
-
- Salonen R, Ashford EA, Gibbs M, Hassani H. Patient preference for oral sumatriptan 25 mg, 50 mg, or 100 mg in the acute treatment of migraine: a double‐blind, randomized, crossover study. Sumatriptan Tablets S2CM11 Study Group. International Journal of Clinical Practice. Supplement 1999;105:16‐24. - PubMed
Savani 2001 {published data only}
-
- Savani N, Pfaffenrath V, Rice L, Boswell D, Black L, Jones M. Sumatriptan SUMB4007 Study Group. Efficacy, tolerability, and patient satisfaction with 50‐ and 100‐mg sumatriptan tablets in those initially dissatisfied with the efficacy of 50‐mg sumatriptan tablets. Clinical Therapeutics 2001;23(2):260‐71. [DOI: 10.1016/S0149-2918(01)80008-4] - DOI - PubMed
Scott 1996 {published data only}
-
- Scott RJ, Aitchison WR, Barker PR, McLaren GI. Oral sumatriptan in the acute treatment of migraine and migraine recurrence in general practice. QJM 1996;89(8):613‐22. - PubMed
Sramek 1999 {published data only}
-
- Sramek JJ, Hussey EK, Clements B, Cutler NR. Oral sumatriptan pharmacokinetics in the migraine state. Clinical Drug Investigation 1999;17(2):137‐44.
SUMA4014 {unpublished data only}
-
- A double‐blind, placebo‐controlled, parallel group study to evaluate the efficacy of a second sumatriptan succinate tablet (25 or 50 mg) in the acute treatment of migraine. www.gsk‐clinicalstudyregister.com/ 1998.
Tepper 2006 {published data only}
Tfelt‐Hansen 2000 {published data only}
-
- Tfelt‐Hansen P. The effectiveness of combined oral lysine acetylsalicylate and metoclopramide (Migpriv) in the treatment of migraine attacks. Comparison with placebo and oral sumatriptan. Functional Neurology 2000;15 Suppl 3:196‐201. - PubMed
Wells 2001 {published data only}
-
- Wells NE. Comparison of the effectiveness of eletriptan, sumatriptan and Cafergot in reducing the time loss associated with migraine attacks. Journal of Medical Economics 2001;4:157‐66.
Wells 2003 {published data only}
-
- Wells N, Hettiarachchi J, Drummond M, Carter D, Parpia T, Pang F. A cost‐effectiveness analysis of eletriptan 40 and 80 mg versus sumatriptan 50 and 100 mg in the acute treatment of migraine. Value Health 2003;6(4):438‐47. - PubMed
Additional references
Bigal 2008
Clarke 1996
-
- Clarke CE, MacMillan L, Sondhi S, Wells NEJ. Economic and social impact of migraine. Quarterly Journal of Medicine 1996;89(1):77‐84. - PubMed
Cook 1995
Derry (forthcoming)
Derry 2012a
Derry 2012b
Derry 2012c
Derry 2012d
Diamond 2007
-
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355‐63. [DOI: 10.1111/j.1526-4610.2006.00631.x] - DOI - PubMed
Edmeads 1993
-
- Edmeads J, Findlay H, Tugwell P, Pryse‐Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension‐type headache on life‐style, consulting behaviour, and medication use: a Canadian population survey. Canadian Journal of Neurological Sciences 1993;20(2):131‐7. - PubMed
Ferrari 1998
-
- Ferrari MD. The economic burden of migraine to society. Pharmacoeconomics 1998;13(6):667‐76. - PubMed
Ferrari 2001
-
- Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5‐HT(1B/1D) agonists) in acute migraine treatment: a meta‐analysis of 53 trials. Lancet 2001;358:1668‐75. - PubMed
Ferrari 2002
Gawel 2001
-
- Gawel MJ, Worthington I, Maggisano A. A systematic review of the use of triptans in acute migraine. Canadian Journal of Neurological Sciences 2001;28(1):30‐41. - PubMed
Gendolla 2008
-
- Gendolla A. Early treatment in migraine: how strong is the current evidence?. Cephalalgia 2008;28 Suppl 2:28‐35. - PubMed
Goadsby 2007
Hazard 2009
Hu 1999
-
- Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Archives of Internal Medicine 1999;159(8):813‐8. - PubMed
IHS 1988
-
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1‐96. - PubMed
IHS 2000
-
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765‐86. - PubMed
IHS 2004
-
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1‐160. - PubMed
Jadad 1996a
-
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. 1996 1996;66(2‐3):239‐46. - PubMed
Jadad 1996b
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. - PubMed
Jhingran 1996
-
- Jhingran P, Cady RK, Rubino J, Miller D, Grice RB, Gutterman DL. Improvements in health‐related quality of life with sumatriptan treatment for migraine. Journal of Family Practice 1996;42(1):36‐42. - PubMed
Kirthi 2010
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. - PubMed
Law 2010
Lipton 1999
-
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy?. Headache 1999;39 Suppl 2:S20‐6.
Lipton 2007
-
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, AMPP Advisory Group, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343‐9. - PubMed
Lofland 1999
-
- Lofland JH, Johnson NE, Batenhorst AS, Nash DB. Changes in resource use and outcomes for patients with migraine treated with sumatriptan: a managed care perspective. Archives of Internal Medicine 1999;159(8):857‐63. - PubMed
Lucas 2006
McCrory 2003
Moore 1998
-
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. - PubMed
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24.
Moore 2010
-
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ‘‘Evidence” in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010;150:386–9. - PubMed
Morris 1995
Oldman 2002
-
- Oldman AD, Smith LA, McQuay HJ, Moore RA. Pharmacological treatments for acute migraine: quantitative systematic review. Pain 2002;97(3):247‐57. - PubMed
Osterhaus 1994
-
- Osterhaus JT, Townsend RJ, Gandek B, Ware JE Jr. Measuring the functional status and well‐being of patients with migraine headache. Headache 1994;34(6):337‐43. - PubMed
Pascual 2002
PCA 2011
-
- Anonymous. Prescription cost analysis, England 2010. The NHS Information Centre, Prescribing Support Unit 2011. [ISBN: 978‐1‐84636‐542‐3]
Radtke 2009
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Solomon 1997
-
- Solomon GD, Price KL. Burden of migraine. A review of its socioeconomic impact. Pharmacoeconomics 1997;11 Suppl 1:1‐10. - PubMed
Stovner 2010
Tramer 1997
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

