Lacosamide for neuropathic pain and fibromyalgia in adults
- PMID: 22336864
- PMCID: PMC8406928
- DOI: 10.1002/14651858.CD009318.pub2
Lacosamide for neuropathic pain and fibromyalgia in adults
Abstract
Background: Antiepileptic drugs have been used in pain management since the 1960s; some seem to be especially useful for neuropathic pain. Lacosamide is an antiepileptic drug that has recently been investigated for neuropathic pain relief, although it failed to get approval for painful diabetic peripheral neuropathy from either the Food and Drug Administration or the European Medicines Agency.
Objectives: To evaluate the analgesic efficacy and adverse effects of lacosamide in the management of chronic neuropathic pain or fibromyalgia.
Search methods: We searched the Cochrane Neuromuscular Disease Group Specialized Register (2011, Issue 4), CENTRAL (2011, Issue 3), MEDLINE (January 2000 to August 2011) and EMBASE (2000 to August 2011) without language restriction, together with reference lists of retrieved papers and reviews.
Selection criteria: We included randomised, double-blind studies of eight weeks duration or longer, comparing lacosamide with placebo or another active treatment in chronic neuropathic pain or fibromyalgia.
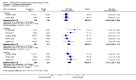
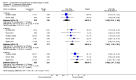
Data collection and analysis: Two review authors independently extracted data for efficacy and adverse events and examined issues of study quality, including risk of bias assessments. Where possible, we calculated numbers needed to treat to benefit from dichotomous data for effectiveness, adverse events and study withdrawals.
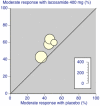
Main results: We included six studies; five (1863 participants) in painful diabetic neuropathy (PDN) and one (159 participants) in fibromyalgia. All were placebo-controlled and titrated to a target dose of 200 mg, 400 mg or 600 mg lacosamide daily, given as a divided dose. Study reporting quality was generally good, although the imputation method of last observation carried forward used in analyses of the primary outcomes is known to known to impart major bias where, as here, adverse event withdrawal rates were high. This, together with small numbers of patients and events for most outcomes at most doses meant that most results were of low quality, with moderate quality evidence available for some efficacy outcomes for 400 mg lacosamide.There were too few data for analysis of the 200 mg dose for painful diabetic neuropathy or any dose for fibromyalgia.In painful diabetic neuropathy, lacosamide 400 mg provided statistically increased rates of achievement of "moderate" and "substantial" benefit (at least 30% and at least 50% reduction from baseline in patient-reported pain respectively) and the patient global impression of change outcome of "much or very much improved". In each case the extra proportion benefiting above placebo was about 10%, yielding numbers needed to treat to benefit compared with placebo of 10 to 12. For lacosamide 600 mg there was no consistent benefit over placebo.There was no significant difference between any dose of lacosamide and placebo for participants experiencing any adverse event or a serious adverse event, but adverse event withdrawals showed a significant dose response. The number needed to treat to harm for adverse event withdrawal was 11 for lacosamide 400 mg and 4 for the 600 mg dose.
Authors' conclusions: Lacosamide has limited efficacy in the treatment of peripheral diabetic neuropathy. Higher doses did not give consistently better efficacy, but were associated with significantly more adverse event withdrawals. Where adverse event withdrawals are high with active treatment compared with placebo and when last observation carried forward imputation is used, as in some of these studies, significant overestimation of treatment efficacy can result. It is likely, therefore, that lacosamide is without any useful benefit in treating neuropathic pain; any positive interpretation of the evidence should be made with caution if at all.
Conflict of interest statement
SD and LH have no known conflicts of interest. RAM has consulted for various pharmaceutical companies and received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions, including (in the past five years) AstraZeneca, Eli Lilly, Flynn Pharma, Futura Medical, Grünenthal, GlaxoSmithKline, Horizon Pharma, Lundbeck, Menarini, MSD, Pfizer, Sanofi Aventis, Urgo, and Vifor Pharma. No author has any financial or other connection with UCB Pharma, which manufactures lacosamide, nor had any involvement in clinical trials of the drug.
Figures

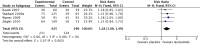









Update of
References
References to studies included in this review
NCT00350103 {published data only}
-
- NCT00350103. A multi‐center, randomized, double‐blind, placebo‐controlled trial to assess the efficacy and safety of 400mg/day lacosamide in subjects with painful distal diabetic neuropathy using two different titration schemes. www.clinicalstudyresults.org/drugdetails/?unique_id=SP874&sort=c.com... (Accessed 18 Aug 2011).
NCT00401830 {published data only}
-
- NCT00401830. Assessing efficacy and safety of lacosamide compared to placebo in reducing signs and symptoms of fibromyalgia syndrome. clinicaltrials.gov/ct2/show/results/NCT00401830?term=NCT00401830&rank=1 (Accessed 18 August 2011).
Rauck 2007 {published data only}
-
- Rauck RL, Shaibani A, Biton V, Simpson J, Koch B. Lacosamide in painful diabetic peripheral neuropathy: a phase 2 double‐blind placebo‐controlled study. Clinical Journal of Pain 2007;23(2):150‐8. [PUBMED: 17237664] - PubMed
Shaibani 2009a {published data only}
-
- Shaibani A, Fares S, Selam JL, Arslanian A, Simpson J, Sen D, Bongardt S. Lacosamide in painful diabetic neuropathy: an 18‐week double‐blind placebo‐controlled trial. Journal of Pain 2009;10(8):818‐28. [PUBMED: 19409861] - PubMed
Wymer 2009 {published data only}
-
- Wymer JP, Simpson J, Sen D, Bongardt S. Efficacy and safety of lacosamide in diabetic neuropathic pain: an 18‐week double‐blind placebo‐controlled trial of fixed‐dose regimens. Clinical Journal of Pain 2009;25(5):376‐85. [PUBMED: 19454870] - PubMed
References to studies excluded from this review
NCT00681068 {published data only}
-
- NCT00681068. A multi‐centre, randomized, double‐blind, placebo controlled pilot trial to assess the efficacy, safety, and tolerability of SPM 927 in subjects with postherpetic neuralgia (PHN). clinicaltrials.gov/ct2/show/study/NCT00861068?term=SPM+927&rank=4 (Accessed 18 August 2011).
Shaibani 2009b {published data only}
-
- Shaibani A, Biton V, Rauck R, Koch B, Simpson J. Long‐term oral lacosamide in painful diabetic neuropathy: a two‐year open‐label extension trial. European Journal of Pain 2009;13(5):458‐63. - PubMed
Additional references
Beydoun 2009
Beyreuther 2006
Beyreuther 2007
Birse 2011
Bouhassira 2008
Corrigan 2011
Dworkin 2008
Dworkin 2010
Errington 2008
Gill 2011
Gustorff 2008
Hall 2006
Hall 2008
Harris 2009
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jensen 2009
Jensen 2011
-
- Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, Treede RD. A new definition of neuropathic pain. Pain 2011;152(10):2204‐5. - PubMed
Kim 2011
-
- Kim Y. Missing data handling in chronic pain trials. Journal of Biopharmaceutical Statistics 2011;21(2):311‐325. - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. - PubMed
McCleane 2009
-
- McCleane G. Lacosamide for pain. Expert Opinion on Investigational Drugs 2010;19(9):1129‐34. - PubMed
McQuay 1997
-
- McQuay HJ, Moore RA. Using numerical results from systematic reviews in clinical practice. Annals of Internal Medicine 1997;126(9):712‐20. - PubMed
McQuay 2007
-
- McQuay HJ, Smith LA, Moore RA. Chronic pain. In: Stevens A, Raftery J, Mant J, Simpson S editor(s). Health care needs assessment. Oxford: Radcliffe Publishing Ltd, 2007:519‐600.
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2009a
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: ] - PMC - PubMed
Moore 2009b
Moore 2010a
-
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: ] - PubMed
Moore 2010b
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: ] - PMC - PubMed
Moore 2010c
-
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers‐needed‐to‐treat analyses‐‐do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo‐controlled chronic low back pain trials. Pain 2010;151(3):592‐7. [DOI: ] - PubMed
Moore 2010d
-
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. [DOI: ] - PubMed
Moore 2011a
Moore 2011b
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: ] - PubMed
Moore 2011c
Moore 2012
-
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al: for the ACTINPAIN writing group of the IASP Special Interest Group (SIG) on Systematic Reviews in Pain Relief and the Cochrane Pain, Palliative and Supportive Care Systematic Review Group editors. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265‐8. [DOI: 10.1016/j.pain.2011.10.004] - DOI - PubMed
NCT00220337
-
- NCT00220337. A trial to assess the long‐term safety and efficacy of lacosamide in subjects with painful diabetic neuropathy. clinicaltrials.gov/ct2/show/NCT00220337?term=lacosamide+AND+pain&rank=2 (Accessed 25 August 2011).
NCT00237458
-
- NCT00237458. An open‐label continuation trial to assess the continued efficacy and safety of ascending doses of lacosamide in subjects with chronic refractory neuropathic pain. clinicaltrials.gov/ct2/show/NCT00237458?term=lacosamide+AND+pain&rank=1 (Accessed 25 August 2011).
NCT00546351
-
- NCT00546351. Open‐label, follow‐on trial to assess the long‐term safety and efficacy of lacosamide in subjects with painful distal diabetic neuropathy. clinicaltrials.gov/ct2/show/NCT00546351?term=lacosamide+AND+pain&rank=3 (Accessed 26 August 2011).
O'Brien 2010
O'Connor 2010
-
- O'Connor AB. LOCF approach to handling missing data overestimates the pain score improvement of drop‐outs. Journal of Pain 2010;11(5):500‐1. [DOI: ] - PubMed
Robinson 2011
Saarto 2007
Sheets 2008
-
- Sheets PL, Heers C, Stoehr T, Cummins TR. Differential block of sensory neuronal voltage‐gated sodium channels by lacosamide [(2R)‐2‐(acetylamino)‐N‐benzyl‐3‐ methoxypropanamide], lidocaine, and carbamazepine. The Journal of Pharmacology and Experimental Therapeutics 2008;326(1):89‐99. [DOI: 10.1124/jpet.107.133413] - DOI - PubMed
Straube 2008
-
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] - DOI - PMC - PubMed
Straube 2010
Sultan 2008
Torrance 2006
Wiffen 2005
Wiffen 2011a
Wiffen 2011b
Wolfe 1990
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33(2):160‐72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

