Sumatriptan (intranasal route of administration) for acute migraine attacks in adults
- PMID: 22336867
- PMCID: PMC4164476
- DOI: 10.1002/14651858.CD009663
Sumatriptan (intranasal route of administration) for acute migraine attacks in adults
Abstract
Background: Migraine is a highly disabling condition for the individual and also has wide-reaching implications for society, healthcare services, and the economy. Sumatriptan is an abortive medication for migraine attacks, belonging to the triptan family. Intranasal administration may be preferable to oral for individuals experiencing nausea and/or vomiting, although it is primarily absorbed in the gut, not the nasal mucosa.
Objectives: To determine the efficacy and tolerability of intranasal sumatriptan compared to placebo and other active interventions in the treatment of acute migraine attacks in adults.
Search methods: We searched Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, online databases, and reference lists for studies through 13 October 2011.
Selection criteria: We included randomised, double-blind, placebo- and/or active-controlled studies using intranasal sumatriptan to treat a migraine headache episode, with at least 10 participants per treatment arm.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. We used numbers of participants achieving each outcome to calculate relative risk (or 'risk ratio') and numbers needed to treat to benefit (NNT) or harm (NNH) compared to placebo or a different active treatment.
Main results: Twelve studies (4755 participants) compared intranasal sumatriptan with placebo or an active comparator. Most of the data were for the 10 mg and 20 mg doses. Sumatriptan surpassed placebo for all efficacy outcomes. For sumatriptan 10 mg versus placebo the NNTs were 7.3, 7.4, and 5.5 for pain-free at two hours, and headache relief at one and two hours, respectively. For sumatriptan 20 mg versus placebo the NNTs were 4.7, 4.9, and 3.5, respectively, for the same outcomes. The 20 mg dose was significantly better than the 10 mg dose for each of these three primary efficacy outcomes.Relief of headache-associated symptoms, including nausea, photophobia, and phonophobia, was greater with sumatriptan than with placebo, and use of rescue medication was lower with sumatriptan than placebo. For the most part, adverse events were transient and mild and were more common with sumatriptan than placebo.Direct comparison of sumatriptan with active treatments was limited to two studies, one comparing sumatriptan 20 mg and dihydroergotamine (DHE) 1 mg, and one comparing sumatriptan 20 mg with rizatriptan 10 mg.
Authors' conclusions: Intranasal sumatriptan is effective as an abortive treatment for acute migraine attacks, relieving pain, nausea, photophobia, phonophobia, and functional disability, but is associated with increased adverse events compared with placebo.
Conflict of interest statement
RAM and SD have received research support from charities, government, and industry sources at various times. RAM has consulted for various pharmaceutical companies, including GlaxoSmithKline, the manufacturers of sumatriptan. RAM has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. GlaxoSmithKline were not in any way involved in this review.
Figures
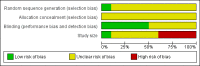


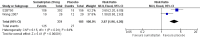
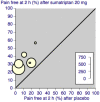
























References
References to studies included in this review
Boureau 2000 {published data only}
-
- Glaxo Study No. S2B‐T60. Cited in Dahlöf 1999, Table 1. Reference given as "Glaxo Study No. S2B‐T60. Data on file with Glaxo Wellcome Research and Development".
-
- Boureau F, Kappos L, Schoenen J, Esperanca P, Ashford E. A clinical comparison of sumatriptan nasal spray and dihydroergotamine nasal spray in the acute treatment of migraine. International Journal of Clinical Practice 2000;54(5):281‐6. [PUBMED: 10954953] - PubMed
Diamond 1998 {published data only}
-
- Diamond S, Elkind A, Jackson RT, Ryan R, DeBussey S, Asgharnejad M. Multiple‐attack efficacy and tolerability of sumatriptan nasal spray in the treatment of migraine. Archives of Family Medicine 1998;7(3):234‐40. [DOI: ] - PubMed
Djupesland 2010 {published data only}
-
- Djupesland PG, Docekal P, Czech Migraine Investigators Group. Intranasal sumatriptan powder delivered by a novel breath‐actuated bi‐directional device for the acute treatment of migraine: a randomised, placebo‐controlled study. Cephalalgia 2010;30(8):933‐42. - PubMed
Peikert 1999 {published data only}
-
- Becker WJ. Glaxo Study No. S2B‐T47. Cited in Dahlöf 1999, Table 1. Reference given as "Becker WJ, on behalf of the study group. A placebo‐controlled, dose‐defining study of sumatriptan nasal spray in the acute treatment of migraine. In: Olesen J, Tfelt‐Hansen P, editors. Headache treatment: trial methodology and new drugs. Philadelphia: Lippincott‐Raven, 1997".
-
- Peikert A, Becker WJ, Ashford EA, Dahlof C, Hassani H, Salonen RJ. Sumatriptan nasal spray: a dose‐ranging study in the acute treatment of migraine. European Journal of Neurology 1999;6(1):43‐9. - PubMed
Ryan 1997 {published data only}
-
- Ryan R, Elkind A, Baker CC, Mullican W, DeBussey S, Asgharnejad M. Sumatriptan nasal spray for the acute treatment of migraine. Results of two clinical studies. Neurology 1997;49(5):1225‐30. - PubMed
S2BT50 {unpublished data only}
-
- A double‐blind, placebo‐controlled, parallel‐group study to evaluate two dose levels (10 mg and 20 mg) of sumatriptan nasal spray in the acute treatment of a migraine attack with an optional repeat dose for headache recurrence. www.gsk‐clinicalstudyregister.com/.
Salonen 1991 {published data only}
-
- The Finnish Sumatriptan Group and the Cardiovascular Clinical Research Group. A placebo‐controlled study of intranasal sumatriptan for the acute treatment of migraine. European Neurology 1991;31(5):332‐8. - PubMed
Salonen 1994 {published data only}
-
- Salonen R, Ashford E, Dahlöf C, Dawson R, Gilhus NE, Lüben V, et al. International Intranasal Sumatriptan Study Group. Intranasal sumatriptan for the acute treatment of migraine. Journal of Neurology 1994;241(8):463‐9. - PubMed
SUM40031 {unpublished data only}
-
- A randomised double‐blind, double‐dummy, single‐attack, parallel group study to compare the speed of onset of sumatriptan nasal spray (20 mg) with rizatriptan wafer (10 mg) in he acute treatment of migraine. www.gsk‐clinicalstudyregister.com/.
Wang 2007 {published data only}
-
- Wang SJ, Fuh JL, Wu ZA. Intranasal sumatriptan study with high placebo response in Taiwanese patients with migraine. Journal of the Chinese Medical Association 2007;70(2):39‐46. - PubMed
References to studies excluded from this review
S2CM12 {unpublished data only}
-
- Glaxo Study No. S2CM12. Cited in Dahlöf 1999, Table 1. Reference given as "Glaxo Study No. S2CM12. Data on file with Glaxo Wellcome Research and Development".
Additional references
Bigal 2008
Clarke 1996
-
- Clarke CE, MacMillan L, Sondhi S, Wells NEJ. Economic and social impact of migraine. Quarterly Journal of Medicine 1996;89(1):77‐84. - PubMed
Cook 1995
Dahlöf 1999
-
- Dahlöf C. Sumatriptan nasal spray in the acute treatment of migraine: a review of clinical studies. Cephalalgia 1999;19(9):769‐78. - PubMed
Derry (forthcoming)
Derry 2012a
Derry 2012b
Derry 2012c
Derry 2012d
Diamond 2007
-
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355‐63. [DOI: 10.1111/j.1526-4610.2006.00631.x] - DOI - PubMed
Edmeads 1993
-
- Edmeads J, Findlay H, Tugwell P, Pryse‐Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension‐type headache on life‐style, consulting behaviour, and medication use: a Canadian population survey. Canadian Journal of Neurological Sciences 1993;20(2):131‐7. - PubMed
Ferrari 1998
-
- Ferrari MD. The economic burden of migraine to society. Pharmacoeconomics 1998;13(6):667‐76. - PubMed
Ferrari 2002
Gendolla 2008
-
- Gendolla A. Early treatment in migraine: how strong is the current evidence?. Cephalalgia 2008;28 Suppl 2:28‐35. - PubMed
Goadsby 2007
Hazard 2009
Hu 1999
-
- Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Archives of Internal Medicine 1999;159(8):813‐8. - PubMed
IHS 1988
-
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1‐96. - PubMed
IHS 2000
-
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765‐86. - PubMed
IHS 2004
-
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1‐160. - PubMed
Jadad 1996a
-
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. - PubMed
Jadad 1996b
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. - PubMed
Jhingran 1996
-
- Jhingran P, Cady RK, Rubino J, Miller D, Grice RB, Gutterman DL. Improvements in health‐related quality of life with sumatriptan treatment for migraine. Journal of Family Practice 1996;42(1):36‐42. - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. - PubMed
Lipton 1999
-
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy?. Headache 1999;39 Suppl 2:S20‐6.
Lipton 2007
-
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, AMPP Advisory Group, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343‐9. - PubMed
Lofland 1999
-
- Lofland JH, Johnson NE, Batenhorst AS, Nash DB. Changes in resource use and outcomes for patients with migraine treated with sumatriptan: a managed care perspective. Archives of Internal Medicine 1999;159(8):857‐63. - PubMed
Lucas 2006
McCrory 2003
Moore 1998
-
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. - PubMed
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24.
Moore 2010
-
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ‘‘Evidence” in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010;150:386–9. - PubMed
Morris 1995
Oldman 2002
-
- Oldman AD, Smith LA, McQuay HJ, Moore RA. Pharmacological treatments for acute migraine: quantitative systematic review. Pain 2002;97(3):247‐57. - PubMed
Osterhaus 1994
-
- Osterhaus JT, Townsend RJ, Gandek B, Ware JE Jr. Measuring the functional status and well‐being of patients with migraine headache. Headache 1994;34(6):337‐43. - PubMed
Pascual 2002
PCA 2011
-
- Anonymous. Prescription cost analysis, England 2010. The NHS Information Centre, Prescribing Support Unit 2011. [ISBN: 978‐1‐84636‐542‐3]
Radtke 2009
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Solomon 1997
-
- Solomon GD, Price KL. Burden of migraine. A review of its socioeconomic impact. Pharmacoeconomics 1997;11 Suppl 1:1‐10. - PubMed
Stovner 2010
Tramer 1997
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

