Sumatriptan (rectal route of administration) for acute migraine attacks in adults
- PMID: 22336868
- PMCID: PMC4170908
- DOI: 10.1002/14651858.CD009664
Sumatriptan (rectal route of administration) for acute migraine attacks in adults
Abstract
Background: Migraine is a highly disabling condition for the individual and also has wide-reaching implications for society, healthcare services, and the economy. Sumatriptan is an abortive medication for migraine attacks, belonging to the triptan family. Rectal administration may be preferable to oral for individuals experiencing nausea and/or vomiting.
Objectives: To determine the efficacy and tolerability of rectal sumatriptan compared to placebo and other active interventions in the treatment of acute migraine attacks in adults.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, online databases, and reference lists for studies through 13 October 2011.
Selection criteria: We included randomised, double-blind, placebo- and/or active-controlled studies using rectally administered sumatriptan to treat a migraine headache episode, with at least 10 participants per treatment arm.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. We used numbers of participants achieving each outcome to calculate relative risk (or 'risk ratio') and numbers needed to treat to benefit (NNT) or harm (NNH) compared to placebo or a different active treatment.
Main results: Three studies (866 participants) compared rectally administered sumatriptan with placebo or an active comparator. Most of the data were for the 12.5 mg and 25 mg doses. For the majority of efficacy outcomes, sumatriptan surpassed placebo. For sumatriptan 12.5 mg versus placebo the NNTs were 5.2 and 3.2 for headache relief at one and two hours, respectively. Results for the 25 mg dose were similar to the 12.5 mg dose, and there were no significant differences between the two doses for any of the outcomes analysed. The NNTs for sumatriptan 25 mg versus placebo were 4.2, 3.2, and 2.4 for pain-free at two hours, headache relief at one hour, and headache relief at two hours, respectively.Relief of functional disability was greater with sumatriptan than with placebo, with NNTs of 8.0 and 4.0 for the 12.5 mg and 25 mg doses, respectively. For the most part, adverse events were transient and mild and were more common with sumatriptan than with placebo, but there were insufficient data to perform any analyses.Direct comparison of sumatriptan with active treatments was limited to one study comparing sumatriptan 25 mg with ergotamine tartrate 2 mg + caffeine 100 mg.
Authors' conclusions: Based on limited amounts of data, sumatriptan 25 mg, administered rectally, is an effective treatment for acute migraine attacks, with participants in these studies experiencing a significant reduction in headache pain and functional disability within two hours of treatment. The lack of data on relief of headache-associated symptoms or incidence of adverse events limits any conclusions that can be drawn.
Conflict of interest statement
RAM and SD have received research support from charities, government, and industry sources at various times. RAM has consulted for various pharmaceutical companies, including GlaxoSmithKline, the manufacturers of sumatriptan, and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. CD has no interests to declare.
GlaxoSmithKline were not in any way involved in carrying out this review.
Figures


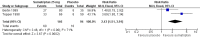










References
References to studies included in this review
Bertin 1999 {published data only}
-
- Bertin L, Brion N, Färkkilä M, Gäbel H, Wessely P. A dose‐defining study of sumatriptan suppositories in the acute treatment of migraine. International Journal of Clinical Practice 1999;53(8):593‐8. [PUBMED: 10692752] - PubMed
S2BT56 {unpublished data only}
-
- A randomised, double‐blind, cross‐over study to compare the efficacy and safety of sumatriptan 25 mg suppository with Cafergot suppository (2 mg ergotamine tartrate, 100 mg caffeine) (one suppository plus option of one additional suppository) in the acute treatment of migraine. www.gsk‐clinicalstudyregister.com/ 1995.
Tepper 1998 {published data only}
-
- Tepper SJ, Cochran A, Hobbs S, Woessner M, S2B351 Study Group. Sumatriptan suppositories for the acute treatment of migraine. International Journal of Clinical Practice 1998;52(1):31‐5. - PubMed
References to studies excluded from this review
Di Monda 2003 {published data only}
-
- Monda V, Nicolodi M, Aloisio A, Bianco P, Fonzari M, Grazioli I, et al. Efficacy of a fixed combination of indomethacin, prochlorperazine, and caffeine versus sumatriptan in acute treatment of multiple migraine attacks: a multicenter, randomized, crossover trial. Headache 2003;43(8):835‐44. [DOI: 10.1046/j.1526-4610.2003.03161.x] - DOI - PubMed
Additional references
Bigal 2008
Clarke 1996
-
- Clarke CE, MacMillan L, Sondhi S, Wells NEJ. Economic and social impact of migraine. Quarterly Journal of Medicine 1996;89(1):77‐84. - PubMed
Cook 1995
Dahlof 2001
-
- Dahlof C. Clinical efficacy and tolerability of sumatriptan tablet and suppository in the acute treatment of migraine: a review of data from clinical trials. Cephalalgia 2001;21 Suppl 1:9‐12. - PubMed
Derry (forthcoming)
Derry 2012a
Derry 2012b
Derry 2012c
Derry 2012d
Diamond 2007
-
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355‐63. [DOI: 10.1111/j.1526-4610.2006.00631.x] - DOI - PubMed
Edmeads 1993
-
- Edmeads J, Findlay H, Tugwell P, Pryse‐Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension‐type headache on life‐style, consulting behaviour, and medication use: a Canadian population survey. Canadian Journal of Neurological Sciences 1993;20(2):131‐7. - PubMed
Ferrari 1998
-
- Ferrari MD. The economic burden of migraine to society. Pharmacoeconomics 1998;13(6):667‐76. - PubMed
Ferrari 2002
Gendolla 2008
-
- Gendolla A. Early treatment in migraine: how strong is the current evidence?. Cephalalgia 2008;28 Suppl 2:28‐35. - PubMed
Goadsby 2007
Hazard 2009
Hu 1999
-
- Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Archives of Internal Medicine 1999;159(8):813‐8. - PubMed
IHS 1988
-
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1‐96. - PubMed
IHS 2000
-
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765‐86. - PubMed
IHS 2004
-
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1‐160. - PubMed
Jadad 1996a
-
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. 1996 1996;66(2‐3):239‐46. - PubMed
Jadad 1996b
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. - PubMed
Jhingran 1996
-
- Jhingran P, Cady RK, Rubino J, Miller D, Grice RB, Gutterman DL. Improvements in health‐related quality of life with sumatriptan treatment for migraine. Journal of Family Practice 1996;42(1):36‐42. - PubMed
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. - PubMed
Lipton 1999
-
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy?. Headache 1999;39 Suppl 2:S20‐6.
Lipton 2007
-
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, AMPP Advisory Group, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343‐9. - PubMed
Lofland 1999
-
- Lofland JH, Johnson NE, Batenhorst AS, Nash DB. Changes in resource use and outcomes for patients with migraine treated with sumatriptan: a managed care perspective. Archives of Internal Medicine 1999;159(8):857‐63. - PubMed
Lucas 2006
McCrory 2003
Moore 1998
-
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. - PubMed
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24.
Moore 2010
-
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ‘‘Evidence” in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010;150:386–9. - PubMed
Morris 1995
Osterhaus 1994
-
- Osterhaus JT, Townsend RJ, Gandek B, Ware JE Jr. Measuring the functional status and well‐being of patients with migraine headache. Headache 1994;34(6):337‐43. - PubMed
Pascual 2002
Radtke 2009
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Solomon 1997
-
- Solomon GD, Price KL. Burden of migraine. A review of its socioeconomic impact. Pharmacoeconomics 1997;11 Suppl 1:1‐10. - PubMed
Stovner 2010
Tramer 1997
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

