The nuclear envelope environment and its cancer connections
- PMID: 22337151
- PMCID: PMC4338998
- DOI: 10.1038/nrc3219
The nuclear envelope environment and its cancer connections
Abstract
Because of the association between aberrant nuclear structure and tumour grade, nuclear morphology is an indispensible criterion in the current pathological assessment of cancer. Components of the nuclear envelope environment have central roles in many aspects of cell function that affect tumour development and progression. As the roles of the nuclear envelope components, including nuclear pore complexes and nuclear lamina, are being deciphered in molecular detail there are opportunities to harness this knowledge for cancer therapeutics and biomarker development. In this Review, we summarize the progress that has been made in our understanding of the nuclear envelope and the implications of changes in this environment for cancer biology.
Figures

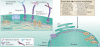

References
-
- Dey P. Cancer nucleus: morphology and beyond. Diagn. Cytopathol. 2010;38:382–390. - PubMed
-
- True LD, Jordan CD. The cancer nuclear microenvironment: interface between light microscopic cytology and molecular phenotype. J. Cell. Biochem. 2008;104:1994–2003. - PubMed
-
- Chatel G, Fahrenkrog B. Nucleoporins: Leaving the nuclear pore complex for a successful mitosis. Cell Signal. 2011 Jun;13 (doi:10.1016/j.cellsig.2011.05.023) - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

