Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor
- PMID: 22337473
- PMCID: PMC3824965
- DOI: 10.1002/syn.21549
Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor
Abstract
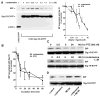
The sigma-1 receptor (Sig-1R) is a novel endoplasmic reticulum (ER) molecular chaperone that regulates protein folding and degradation. The Sig-1R activation by agonists is known to improve memory, promote cell survival, and exert an antidepressant-like action in animals. Cutamesine (SA4503), a selective Sig-1R ligand, was shown to increase BDNF in the hippocampus of rats. How exactly the intracellular chaperone Sig-1R or associated ligand causes the increase of BDNF or any other neurotrophins is unknown. We examined here whether the action of Sig-1Rs may relate to the post-translational processing and release of BDNF in neuroblastoma cell lines. We used in vitro assays and confirmed that cutamesine possesses the bona fide Sig-1R agonist property by causing the dissociation of BiP from Sig-1Rs. The C-terminus of Sig-1Rs exerted robust chaperone activity by completely blocking the aggregation of BDNF and GDNF in vitro. Chronic treatment with cutamesine in rat B104 neuroblastoma caused a time- and dose-dependent potentiation of the secretion of BDNF without affecting the mRNA level of BDNF. Cutamesine decreased the intracellular level of pro-BDNF and mature BDNF whereas increased the extracellular level of mature BDNF. The pulse-chase experiment indicated that the knockdown of Sig-1Rs decreased the secreted mature BDNF in B104 cells without affecting the synthesis of BDNF. Our findings indicate that, in contrast to clinically used antidepressants that promote the transcriptional upregulation of BDNF, the Sig-1R agonist cutamesine potentiates the post-translational processing of neurotrophins. This unique pharmacological profile may provide a novel therapeutic opportunity for the treatment of neuropsychiatric disorders.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
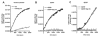



Similar articles
-
Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB.J Pharmacol Exp Ther. 2010 Feb;332(2):388-97. doi: 10.1124/jpet.109.160960. Epub 2009 Oct 23. J Pharmacol Exp Ther. 2010. PMID: 19855099 Free PMC article.
-
Sigma-1 receptor agonist PRE-084 increases BDNF by activating the ERK/CREB pathway to rescue learning and memory impairment caused by type II diabetes.Behav Brain Res. 2025 Apr 27;484:115493. doi: 10.1016/j.bbr.2025.115493. Epub 2025 Feb 20. Behav Brain Res. 2025. PMID: 39986614
-
The sigma-1 receptor chaperone as an inter-organelle signaling modulator.Trends Pharmacol Sci. 2010 Dec;31(12):557-66. doi: 10.1016/j.tips.2010.08.007. Epub 2010 Oct 1. Trends Pharmacol Sci. 2010. PMID: 20869780 Free PMC article.
-
The Role of Sigma-1 Receptor, an Intracellular Chaperone in Neurodegenerative Diseases.Curr Neuropharmacol. 2018;16(1):97-116. doi: 10.2174/1570159X15666170529104323. Curr Neuropharmacol. 2018. PMID: 28554311 Free PMC article. Review.
-
The sigma-1 receptor: roles in neuronal plasticity and disease.Trends Neurosci. 2012 Dec;35(12):762-71. doi: 10.1016/j.tins.2012.09.007. Epub 2012 Oct 23. Trends Neurosci. 2012. PMID: 23102998 Free PMC article. Review.
Cited by
-
3,4-Methylenedioxy methamphetamine, synthetic cathinones and psychedelics: From recreational to novel psychotherapeutic drugs.Front Psychiatry. 2022 Oct 3;13:990405. doi: 10.3389/fpsyt.2022.990405. eCollection 2022. Front Psychiatry. 2022. PMID: 36262632 Free PMC article. Review.
-
Discovery of N-cyclobutylaminoethoxyisoxazole derivatives as novel sigma-1 receptor ligands with neurite outgrowth efficacy in cells.RSC Adv. 2018 Feb 14;8(13):7080-7088. doi: 10.1039/c8ra00072g. eCollection 2018 Feb 9. RSC Adv. 2018. PMID: 35540351 Free PMC article.
-
Impairment and Restoration of Homeostatic Plasticity in Cultured Cortical Neurons From a Mouse Model of Huntington Disease.Front Cell Neurosci. 2019 May 16;13:209. doi: 10.3389/fncel.2019.00209. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31156395 Free PMC article.
-
Allosteric Modulation of Sigma-1 Receptors Elicits Rapid Antidepressant Activity.CNS Neurosci Ther. 2016 May;22(5):368-77. doi: 10.1111/cns.12502. Epub 2016 Feb 8. CNS Neurosci Ther. 2016. PMID: 26854125 Free PMC article.
-
Pharmacological Analysis of GABAA Receptor and Sigma1R Chaperone Interaction: Research Report I-Investigation of the Anxiolytic, Anticonvulsant and Hypnotic Effects of Allosteric GABAA Receptors' Ligands.Int J Mol Sci. 2023 May 31;24(11):9580. doi: 10.3390/ijms24119580. Int J Mol Sci. 2023. PMID: 37298532 Free PMC article.
References
-
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. - PubMed
-
- Baj G, Tongiorgi E. BDNF splice variants from the second promoter cluster support cell survival of differentiated neuroblastoma upon cytotoxic stress. J Cell Sci. 2009;122:36–43. - PubMed
-
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11:1169–80. - PubMed
-
- Calabrese F, Molteni R, Cattaneo A, Macchi F, Racagni G, et al. Long-Term duloxetine treatment normalizes altered brain-derived neurotrophic factor expression in serotonin transporter knockout rats through the modulation of specific neurotrophin isoforms. Mol Pharmacol. 2010;77:846–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
