The thrombopoietin/MPL/Bcl-xL pathway is essential for survival and self-renewal in human preleukemia induced by AML1-ETO
- PMID: 22337712
- PMCID: PMC3412344
- DOI: 10.1182/blood-2012-01-403212
The thrombopoietin/MPL/Bcl-xL pathway is essential for survival and self-renewal in human preleukemia induced by AML1-ETO
Abstract
AML1-ETO (AE) is a fusion product of translocation (8;21) that accounts for 40% of M2 type acute myeloid leukemia (AML). In addition to its role in promoting preleukemic hematopoietic cell self-renewal, AE represses DNA repair genes, which leads to DNA damage and increased mutation frequency. Although this latter function may promote leukemogenesis, concurrent p53 activation also leads to an increased baseline apoptotic rate. It is unclear how AE expression is able to counterbalance this intrinsic apoptotic conditioning by p53 to promote survival and self-renewal. In this report, we show that Bcl-xL is up-regulated in AE cells and plays an essential role in their survival and self-renewal. Further investigation revealed that Bcl-xL expression is regulated by thrombopoietin (THPO)/MPL-signaling induced by AE expression. THPO/MPL-signaling also controls cell cycle reentry and mediates AE-induced self-renewal. Analysis of primary AML patient samples revealed a correlation between MPL and Bcl-xL expression specifically in t(8;21) blasts. Taken together, we propose that survival signaling through Bcl-xL is a critical and intrinsic component of a broader self-renewal signaling pathway downstream of AML1-ETO-induced MPL.
Figures

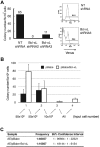


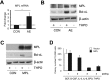
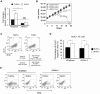

Comment in
-
JAKing up AML1-ETO.Blood. 2012 Jul 26;120(4):703-4. doi: 10.1182/blood-2012-05-413104. Blood. 2012. PMID: 22835885 No abstract available.
References
-
- Look AT. Oncogenic transcription factors in the human acute leukemias. Science. 1997;278(5340):1059–1064. - PubMed
-
- Okuda T, Cai Z, Yang S, et al. Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood. 1998;91(9):3134–3143. - PubMed
-
- Higuchi M, O'Brien D, Kumaravelu P, Lenny N, Yeoh EJ, Downing JR. Expression of a conditional AML1-ETO oncogene bypasses embryonic lethality and establishes a murine model of human t(8;21) acute myeloid leukemia. Cancer Cell. 2002;1(1):63–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

