The embryonic origins of erythropoiesis in mammals
- PMID: 22337720
- PMCID: PMC3367890
- DOI: 10.1182/blood-2012-01-153486
The embryonic origins of erythropoiesis in mammals
Abstract
Erythroid (red blood) cells are the first cell type to be specified in the postimplantation mammalian embryo and serve highly specialized, essential functions throughout gestation and postnatal life. The existence of 2 developmentally and morphologically distinct erythroid lineages, primitive (embryonic) and definitive (adult), was described for the mammalian embryo more than a century ago. Cells of the primitive erythroid lineage support the transition from rapidly growing embryo to fetus, whereas definitive erythrocytes function during the transition from fetal life to birth and continue to be crucial for a variety of normal physiologic processes. Over the past few years, it has become apparent that the ontogeny and maturation of these lineages are more complex than previously appreciated. In this review, we highlight some common and distinguishing features of the red blood cell lineages and summarize advances in our understanding of how these cells develop and differentiate throughout mammalian ontogeny.
Figures
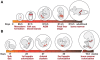



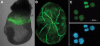
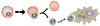

References
-
- Tavian M, Peault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49(2):243–250. - PubMed
-
- Baron MH. Early patterning of the mouse embryo: implications for hematopoietic commitment and differentiation. Exp Hematol. 2005;33(9):1015–1020. - PubMed
-
- Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33(9):1041–1047. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

