Selective TRIF-dependent signaling by a synthetic toll-like receptor 4 agonist
- PMID: 22337809
- PMCID: PMC3684200
- DOI: 10.1126/scisignal.2001963
Selective TRIF-dependent signaling by a synthetic toll-like receptor 4 agonist
Abstract
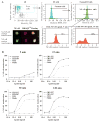
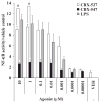
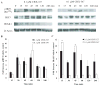
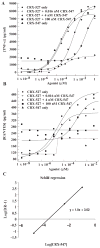
In response to ligand binding to the Toll-like receptor 4 (TLR4) and myeloid differentiation-2 (MD-2) receptor complex, two major signaling pathways are activated that involve different adaptor proteins. One pathway depends on myeloid differentiation marker 88 (MyD88), which elicits proinflammatory responses, whereas the other depends on Toll-IL-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF), which elicits type I interferon production. Here, we showed that the TLR4 agonist and vaccine adjuvant CRX-547, a member of the aminoalkyl glucosaminide 4-phosphate (AGP) class of synthetic lipid A mimetics, displayed TRIF-selective signaling in human cells, which was dependent on a minor structural modification to the carboxyl bioisostere corresponding to the 1-phosphate group on most lipid A types. CRX-547 stimulated little or no activation of MyD88-dependent signaling molecules or cytokines, whereas its ability to activate the TRIF-dependent pathway was similar to that of a structurally related inflammatory AGP and of lipopolysaccharide from Salmonella minnesota. This TRIF-selective signaling response resulted in the production of substantially less of the proinflammatory mediators that are associated with MyD88 signaling, thereby potentially reducing toxicity and improving the therapeutic index of this synthetic TLR4 agonist and vaccine adjuvant.
Conflict of interest statement
Figures

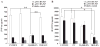
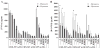
References
-
- Bortolatto J, Borducchi E, Rodriguez D, Keller AC, Faquim-Mauro E, Bortoluci KR, Mucida D, Gomes E, Christ A, Schnyder-Candrian S, Schnyder B, Ryffel B, Russo M. Toll-like receptor 4 agonists adsorbed to aluminium hydroxide adjuvant attenuate ovalbumin-specific allergic airway disease: Role of MyD88 adaptor molecule and interleukin-12/interferon-γ axis. Clin Exp Allergy. 2008;38:1668–1679. - PubMed
-
- Ishizaka ST, Hawkins LD. E6020: A synthetic Toll-like receptor 4 agonist as a vaccine adjuvant. Expert Rev Vaccines. 2007;6:773–784. - PubMed
-
- Johnson DA. Synthetic TLR4-active glycolipids as vaccine adjuvants and stand-alone immunotherapeutics. Curr Top Med Chem. 2008;8:64–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

