Rac1 protein regulates glycogen phosphorylase activation and controls interleukin (IL)-2-dependent T cell proliferation
- PMID: 22337875
- PMCID: PMC3320936
- DOI: 10.1074/jbc.M111.297804
Rac1 protein regulates glycogen phosphorylase activation and controls interleukin (IL)-2-dependent T cell proliferation
Abstract
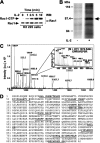
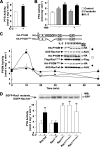
Small GTPases of the Rho family have been implicated in important cellular processes such as cell migration and adhesion, protein secretion, and/or gene transcription. In the lymphoid system, these GTPases participate in the signaling cascades that are activated after engagement of antigen receptors. However, little is known about the role that Rho GTPases play in IL-2-mediated responses. Here, we show that IL-2 induces Rac1 activation in Kit 225 T cells. We identified by mass spectrometry the muscle isoform of glycogen phosphorylase (PYGM) as a novel Rac1 effector molecule in IL-2-stimulated cells. The interaction between the active form of Rac1 (Rac1-GTP) and PYGM was established directly through a domain comprising amino acids 191-270 of PYGM that exhibits significant homology with the Rac binding domain of PAK1. The integrity of this region was crucial for PYGM activation. Importantly, IL-2-dependent cellular proliferation was inhibited upon blocking both the activation of Rac1 and the activity of PYGM. These results reveal a new role for Rac1 in cell signaling, showing that this GTPase triggers T cell proliferation upon IL-2 stimulation by associating with PYGM and modulating its enzymatic activity.
Figures


References
-
- Beier I., Düsing R., Vetter H., Schmitz U. (2008) Epidermal growth factor stimulates Rac1 and p21-activated kinase in vascular smooth muscle cells. Atherosclerosis 196, 92–97 - PubMed
-
- Terakado M., Gon Y., Sekiyama A., Takeshita I., Kozu Y., Matsumoto K., Takahashi N., Hashimoto S. (2011) The Rac1/JNK pathway is critical for EGFR-dependent barrier formation in human airway epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L56–L63 - PubMed
-
- Bäumer A. T., Ten Freyhaus H., Sauer H., Wartenberg M., Kappert K., Schnabel P., Konkol C., Hescheler J., Vantler M., Rosenkranz S. (2008) Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for α-platelet-derived growth factor receptor-induced production of reactive oxygen species. J. Biol. Chem. 283, 7864–7876 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

